September 1, 2010 – The European Society of Cardiology (ESC) upgraded fractional flow reserve (FFR) to a class I, level of evidence A, the highest class and level possible. The guidelines for FFR-guided treatment were included in the updated Guidelines on Percutaneous Coronary Intervention (PCI) announced Monday at the ESC congress in Stockholm.
The ESC guidelines, which are intended to assist healthcare providers in clinical decision-making, now classify FFR-guided treatment as class I, with level of evidence A. Level of evidence A is the highest level available, requiring the most clinical evidence and is awarded only when data has been derived from multiple randomized clinical trials or meta-analyses. Class I indicates a general agreement that a given treatment or procedure is beneficial, useful and effective.
“The overall aim of the new guidelines for both percutaneous and surgical revascularization has been to shift emphasis from using primarily the anatomical expression of coronary artery disease to guide treatment toward a more patient-oriented perspective, where all aspects of the disease are taken into account. In this context, the physiological impact of coronary lesions and FFR measurements play a central role,” said Stefan James, M.D., associate professor of cardiology at Uppsala University Hospital in Uppsala, Sweden, and a member of the ESC guidelines task force for myocardial revascularization.
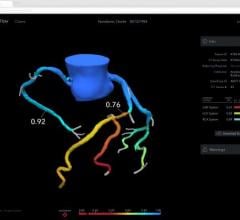
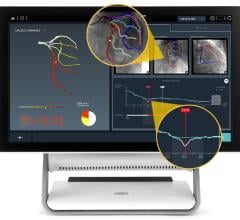
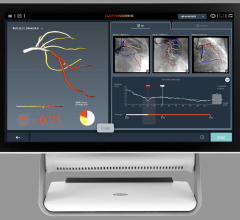
Current data show physiological assessment using FFR prior to placement of coronary stents helps physicians better optimize clinical outcomes by determining which specific lesion or lesions are responsible for a patient’s ischemia, a deficiency of blood supply to the heart caused by blood restriction. The recommendations supported by a level of evidence A state that FFR measurements can be useful in assessing whether an intervention in a coronary lesion is necessary as an alternative to noninvasive functional testing and to help assess intermediate stenosis in patients with anginal symptoms.
Supporting this change are the very strong one- and two-year data from the landmark FAME (Fractional Flow Reserve [FFR] vs. Angiography in Multivessel Evaluation) trial. It demonstrated improved outcomes for patients with multivessel coronary artery disease whose treatment was guided by St. Jude Medical FFR measurement systems rather than by angiography alone.
The FAME study was a randomized, prospective, multi-center trial, which enrolled 1,005 patients with multivessel coronary artery disease. It compared outcomes for patients whose treatment was guided by FFR to those whose treatment was guided only by angiography. The 12-month results, published in the January 15, 2009 issue of the New England Journal of Medicine, demonstrated that instances of major adverse cardiovascular events (MACE), such as death, myocardial infarction or repeat revascularization, were reduced by 30 percent for patients whose treatment was guided by FFR rather than by standard angiography alone.
Two-year FAME results presented as a late-breaking trial during the 2009 Transcatheter Cardiovascular Therapeutics (TCT) Conference demonstrated that patients who received FFR-guided treatment continued to experience improved outcomes over time, including a 34 percent risk reduction of death or myocardial infarction (heart attack). FFR-guided treatment was also demonstrated to be cost-saving, with a difference of about $2,000, or 14 percent, between total healthcare costs for the FFR-guided cohort and the group treated by angiography alone. These lower healthcare costs were a result of reduced procedural costs, reduced follow-up costs for major adverse cardiac events and shorter hospital stays.
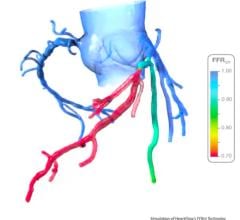
About Fractional Flow Reserve
FFR is an index determining the functional severity of narrowings in the coronary arteries. FFR specifically identifies which coronary narrowings are responsible for significantly obstructing the flow of blood to a patient's heart muscle and it is used by the interventional cardiologist to direct coronary interventions and assess results for improved treatment outcomes.
For more information: sjm.com


 February 03, 2026
February 03, 2026