September 15, 2022 — Today, aortic stenosis (AS) is one of the most common and serious valve disease problems [1]. It narrows the aortic valve opening and restricts the blood flow to the heart, making the heart work harder and potentially causing symptoms including chest pain, shortness of breath and fatigue. Left untreated, severe aortic valve stenosis can lead to death. Many patients with severe symptomatic aortic stenosis (SAS) are treated with a transcatheter aortic valve replacement (TAVR) – a minimally invasive procedure that replaces a diseased aortic valve with a man-made valve. As an alternative to open-heart aortic valve replacement surgery, TAVR treatment offers several benefits for symptomatic SAS patients, including reduced length of hospital stay and an increased likelihood of home discharge [2].
With expanding access and more patients eligible for TAVR, the number of procedures performed is expected to rise significantly. In the U.S. alone, the rate of treatment increased from 6,481 in 2012 to 73,411 in 2019 [3] [4] [5]. However, patients can experience complications for days or weeks after being discharged, which may lead to readmission to the hospital or even death. This makes post-acute monitoring a crucial element to help ensure optimal patient outcomes following the procedure
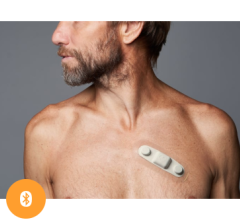
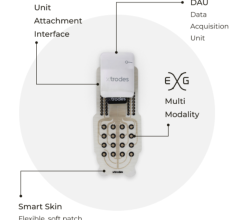
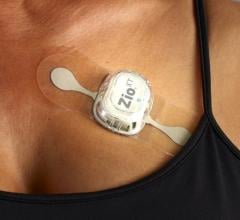
That’s where ambulatory cardiac monitoring services, such as Philips MCOT (Mobile Cardiac Outpatient Telemetry), come in. It continuously analyzes the cardiac rhythm and transmit critical findings, allowing for near real-time detection of clinically significant arrhythmias that may occur with TAVR patients after discharge. To help provide proactive care and improve outcomes, many hospitals have begun to leverage the MCOT patch system technology to monitor for significant arrhythmias beyond the confines of the hospital. As TAVR numbers continue to climb, there is a greater need to understand how these strategies impact patient outcomes and the costs associated with the procedure.
The Value of High-Quality Care
The recent study, “Costs and Outcomes of Mobile Cardiac Outpatient Telemetry Monitoring Post-Transcatheter Aortic Valve Replacement,” published in the August issue of the Journal of Comparative Effectiveness Research, examined the impact that using Philips MCOT had on patient outcomes and value of high-quality care following TAVR procedures. Using a retrospective an claims analysis, the study analyzed pacemaker insertions, length of stay, average costs, payments and contribution margins for patients undergoing TAVR procedures with MCOT monitoring post-procedure, compared to patients with no MCOT monitoring. The study revealed that MCOT-monitored TAVR hospital patients were more than 16% more likely to receive a permanent pacemaker through a scheduled physician referral than unmonitored patients (100% MCOT vs 83.5% non-MCOT), helping to avoid unplanned readmissions [6]. The study illustrates that prescribing MCOT to patients post-TAVR may provide an opportunity for improved health outcomes or cost savings by monitoring for arrhythmic disturbances, and, if detected, allowing for timely intervention via pacemaker and the avoidance of costly and dangerous emergency care.
“Ambulatory monitoring has become a critical component of cardiac care. This new research validates that Philips MCOT is crucial in detecting arrhythmias and providing data that allows care teams to work quickly and act decisively to provide the best patient treatment,” said Andy Broadway, General Manager of Ambulatory Monitoring and Diagnostics at Philips. “There is value in identifying the moment a patient’s condition begins to change and that value is extended even further when the approach achieves all aspects of the Quadruple Aim in healthcare.”
When evaluating cost, Philips MCOT was also associated with significantly lower hospital costs, fewer TAVR patient admissions and improved hospital contribution margins. Even with the increased rate of pacemaker insertion in the MCOT group, there was no observed impact on costs in the 60-day period post-TAVR, suggesting that MCOT provides an opportunity to improve patient care without afflicting greater cost to the hospital or payer [6].
Standards of Care Centered Around the Quadruple Aim
The results from this study and others evaluating the impact of remote monitoring technology play an important role in shaping the standards of care in place today, particularly as clinicians, hospitals, and policymakers continue to look for opportunities to balance the delivery of high-quality care while managing costs.
In another recent Philips study, researchers evaluated MCOT as a first-line diagnostic ambulatory monitoring solution with post-cryptogenic stroke patients and produced similar findings, further supporting MCOT’s value as it relates to cost benefits and improved patient outcomes. The study determined that a 30-day continuous monitoring program using the Philips MCOT, followed by an implantable loop recorder (ILR), improved atrial fibrillation detection rates and helped to reduce secondary stroke risk – validating the approach as an effective standard of care for cryptogenic stroke patients.
By connecting information, technologies and people across both the stroke and cardiac care pathways, healthcare can elevate its standards of care to help achieve the Quadruple Aim. For cardiology, adding monitoring programs into the post-procedural standard of care may provide a cost-neutral solution for addressing the unmet needs in monitoring for delayed cardiac arrhythmias in the TAVR population, ultimately helping to improve patient care.
For more information: www.philips.com
References:
[1] American Heart Association, https://www.heart.org/en/health-topics/heart-valve-problems-and-disease/heart-valve-problems-and-causes/problem-aortic-valve-stenosis
[2] Arora S, Strassle PD, Kolte D, et al. Length of stay and discharge disposition after transcatheter 283 versus surgical aortic valve replacement in the United States. Circ Cardiovasc Interv. 2018;11(9). 284 doi:10.1161/circinterventions.118.006929
[3] Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363(17):1597-607. doi:10.1056/NEJMoa1008232
[4] Makkar RR, Fontana GP, Jilaihawi H, et al. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N Engl J Med. 2012;366(18):1696-704. doi:10.1056/NEJMoa1202277
[5] Carroll JD, Mack MJ, Vemulapalli S, et al. STS-ACC TVT registry of transcatheter aortic valve replacement. J Am Coll Cardiol. 2020;76(21):2492-2516. doi:10.1016/j.jacc.2020.09.595
[6] Belinda A Mohr, PhD1, et al. Impact of Mobile Cardiac Outpatient Telemetry Post-Transcatheter Aortic Valve Replacement on Costs and Outcomes: A Medicare Claims Analysis. J. Comp. Eff. Res. 2022. https://www.futuremedicine.com/doi/10.2217/cer-2022-0112


 January 14, 2026
January 14, 2026