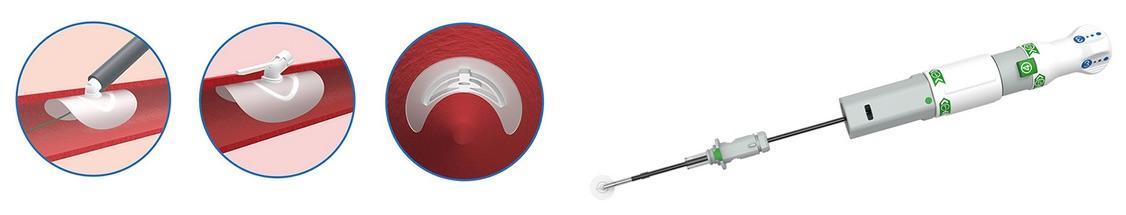
November 21, 2018 – Vivasure Medical Ltd. recently announced the European launch of the PerQseal closure device for large-bore transcatheter procedures.
Novel transcatheter endovascular procedures — including transcatheter aortic valve replacement (TAVR), thoracic endovascular aneurysm repair (TEVAR) and endovascular abdominal aneurysm repair (EVAR) — require large bore femoral artery access. Closure of these large bore access sites is challenging and has been associated with significant vascular and bleeding complications.
PerQseal is the first sutureless, fully absorbable synthetic implant for large bore arterial punctures, according to Vivasure. The PerQseal technology consists of an intravascular patch that seals the vessel from the inside, returning the artery to its natural state.
“Closing the artery has been a concern since we started using transcatheter techniques for valve implantation,” said Prof. Horst Sievert, of the CardioVascular Center in Frankfurt, Germany. “The PerQseal device is a very innovative solution for closing large holes, and we are enthusiastic to make it part of our armamentarium.”
“In my first clinical experience with PerQseal, I found the device intuitive and well controlled, which helped me quickly learn how to use the technology safely and successfully,” said Saib Khogali, M.D., Heart & Lung Centre, New Cross Hospital Wolverhampton, U.K. “I believe the PerQseal has the potential to be an important large hole closure device in many TAVR and EVAR patients.”
For more information: www.vivasuremedical.com


 November 14, 2025
November 14, 2025 









