October 26, 2016 — Philips recently announced its latest image guidance solutions to be featured at the 2016 Transcatheter Cardiovascular Therapeutics meeting (TCT) in Washington, D.C., Oct. 29 – Nov. 2, 2016. Highlights will include the introduction of instant wave-Free Ratio (iFR) co-registration, which integrates iFR pullback data with the angiogram, and the third generation of HeartNavigator, live image guidance software for advanced structural heart disease procedures.
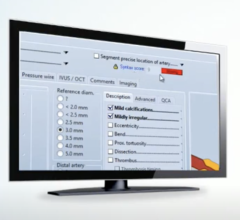
Philips Image Guided Therapy tools help clinicians decide, guide and confirm the right therapy for delivery of better care to help improve patient and economic outcomes. With iFR co-registration, clinicians can assess coronary artery blockages by using the Philips Volcano pressure measurement guidewires and equipment. There is no need for time-consuming pullback devices or guesswork.
Philips will also be introducing the third generation of its HeartNavigator planning and guidance software that provides functionality to measure and select an aortic valve repair device, and choose the X-ray viewing angle. With HeartNavigator, a 3-D volume is rendered from previously acquired computed tomography (CT) datasets and overlaid on live fluoro for real-time 3-D guidance. The software provides an immersive user experience, giving physicians more confidence during challenging procedures such as transcatheter aortic valve replacement (TAVR).
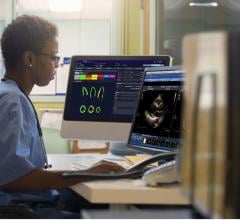
In addition, Philips will also feature key integrated cardiology solutions, including ultrasound imaging and healthcare IT that demonstrate the company's strategy across the health continuum. The company also will host several events and offer live demonstrations, including iFR co-registration, at the Interactive Technology Center to allow attendees to better understand the clinical benefits of its offerings.
Other advanced image-guidance technologies to be highlighted will include:
- IntelliSpace Cardiovascular – designed to help streamline workflow and improve operational performance throughout the cardiovascular care continuum;
- EchoNavigator – fuses live transesophageal echocardiography (TEE) and live X-ray imaging for guiding advanced SHD procedures in cardiac catheter and hybrid labs; and
- Epiq 7 Ultrasound System for Cardiology
During TCT, Philips also will host several on-site events, including:
- Lunch Symposium: The future of physiology – Exploring iFR co-registration and ischemia-guided PCI (percutaneous coronary intervention)
- Interactive Technology Center: Demonstration of iFR and IVUS (intravascular ultrasound) co-registration on SyncVision systems; and
- Dinner Symposium: Controversies in PCI optimization – A case-based discussion
For more information: www.usa.philips.com/healthcare


 November 06, 2025
November 06, 2025