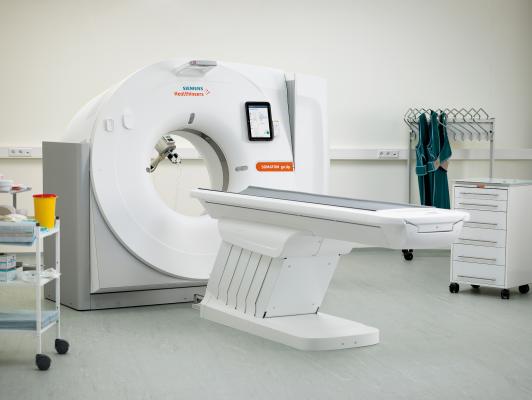
November 15, 2017 — Center for Diagnostic Imaging (CDI), one of the nation’s largest providers of diagnostic imaging services, recently became the first healthcare organization in the United States to install the Somatom go.Up computed tomography (CT) system from Siemens Healthineers. The scanners are installed at two CDI outpatient imaging facilities associated with St. Luke’s Hospital in St. Louis: St. Luke’s CDI Chesterfield, Mo., and St. Luke’s CDI Frontenac, Mo. The Somatom go.Up is designed for highly diverse sets of user needs and provides automated, standardized workflows that help users reduce unwanted variations and achieve more consistent clinical results at a lower total cost of ownership.
A highlight of the system is the user’s ability to control routine examinations utilizing only the tablet and remote. This ability facilitates a new, mobile workflow whereby staff no longer need to move between scanner and control room and can remain with patients during scan preparation, potentially improving the patient experience. Standardized work steps permit users to run the scan with just a few inputs, and automated, zero-click post-processing enables efficient and consistent scanner operation.
Additional features include a wide detector that provides up to 64 slices, enabling more rapid CT scanning. With a combination of advanced technologies such as the Stellar integrated detector, SAFIRE¹ iterative reconstruction and tin filter technology, the system delivers some of the lowest radiation doses achievable for a CT of its class, according to Siemens. The scanner is ideal for institutions that want to expand their imaging portfolio.
For more information: www.usa.healthcare.siemens.com
References
1. In clinical practice, the use of SAFIRE may reduce CT patient dose depending on the clinical task, patient size, anatomical location, and clinical practice. A consultation with a radiologist and a physicist should be made to determine the appropriate dose to obtain diagnostic image quality for the particular clinical task.


 February 02, 2026
February 02, 2026 









