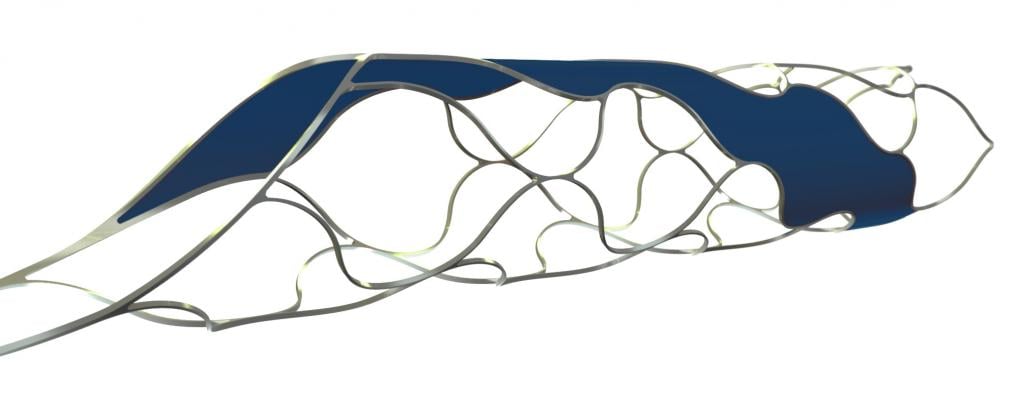
March 8, 2016 — phenox GmbH announced participation in the SITS Open clinical trial, where pREset and pREset LITE thrombectomy devices have been included in the study device portfolio. pREset is one of only three devices to be used in the study.
Stroke is the second leading cause of death worldwide and the most common cause of adult disability in the western world. Ischemic stroke, caused by a blocked artery, is the most common type of stroke and it takes only minutes without oxygen for brain damage to occur and more brain cells die every minute that flow is blocked. The aim of physicians is to restore blood flow by opening the blocked artery as quickly as possible, thereby enhancing the patient’s survivability and preventing long lasting disability.
The positive outcomes of recent randomized clinical trials for mechanical thrombectomy, such as MR CLEAN, ESCAPE, EXTEND IA and SWIFT PRIME, have renewed interest in studies enrolling a large number of patients. The SITS Open protocol is designed to provide a higher level of evidence for mechanical thrombectomy through a direct comparison between mechanical thrombectomy and a concurrent control of medical management alone.
The Department of Neuroscience at Karolinska Institute, Stockholm, Sweden, is sponsoring the open, prospective, international, multicenter, controlled clinical trial. The protocol calls for enrolling 600 patients in total, 300 in each arm. Patients enrolled in the treatment arm will be done so at centers that currently perform thrombectomy for stroke and fulfill the quality and training criteria for neuro-interventions. Patients in the control arm will be enrolled by clinics that offer IV thrombolysis and neither practice thrombectomy nor refer patients with ischemic stroke to other clinics where thrombectomy is offered.
For more information: www.phenox.net


 January 05, 2026
January 05, 2026 









