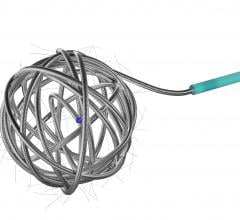
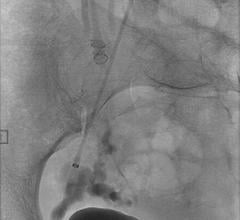
June 23, 2011 — A new study published in the June edition of the Journal of NeuroInterventional Surgery highlights that a more uniform distribution of endovascular coils may help in the treatment of cerebral aneurysms. Deltapaq endovascular microcoils from Codman & Shurtleff Inc., a global neurovascular and neuroscience company, were used in the study.
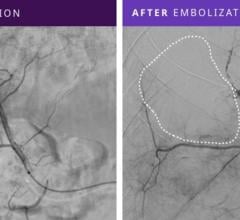
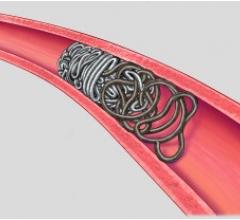
Study authors found that the novel triangular primary wind design of Deltapaq achieved a tighter packing density and a more uniform distribution of the coil mass across the aneurysm dome in comparison with helical and complex endovascular microcoils. Those coils were also more likely to have the highest rate of angiographic occlusion.
Researchers concluded that the evaluation of emerging coil technologies with respect to treatment durability may be well served by an assessment of their uniformity of distribution within an aneurysm, in addition to the traditional packing density and angiographic occlusion scoring methods.
"This study suggests that the uniformity across the dome of the aneurysm may play an important role in enhancing the durability of endovascular treatment and is an important area for further study and confirmation," said Bernard R. Bendok, M.D., study co-author and associate professor of neurological surgery and radiology at Northwestern University's Feinberg School of Medicine. "The application of a coil uniformity model may provide valuable design information."
Study authors constructed silicone aneurysm models and devised an assessment tool to objectively measure the coil uniformity index, which when combined with the degree of angiographic occlusion and packing density can provide valuable information regarding coil behavior.
In the in vitro study, Deltapaq coils were more uniformly distributed in the dome of the aneurysm than the complex and helical coils. The average packing density for Deltapaq (39.1 percent) was also significantly higher than the complex (35.2 percent) and helical (32.2 percent) coil systems. In addition, Deltapaq had the highest rate of angiographic occlusion based on the Raymond score, a qualitative measure of permeability.
The study was funded by a research grant from Micrus Endovascular Inc., which now operates under Codman Neurovascular.
Endovascular coils are inserted directly into an aneurysm through a microcatheter, which could lead to a hemorrhagic stroke, a life-threatening condition that affects about 13 percent of the 800,000 stroke cases each year in the United States. According to the American Stroke Association, cerebral aneurysms affect 3 to 5 million people in the United States.
For more information: www.codman.com


 June 05, 2025
June 05, 2025