July 1, 2010 – Clinical results have shown the Rheos System is successful in lowering blood pressure, CVRx Inc. announced this week at the European Society of Hypertension Meeting. The system is the first device designed to treat hypertension (high blood pressure) and is a future treatment option for the millions of people who cannot control their blood pressure with medications.
Dr. Bram Kroon, associate professor and vascular medicine specialist, Department of Internal Medicine, MUMC+, Maastricht, presented the four-year results from the Device-Based Therapy of Hypertension (DEBuT-HT) study. The findings show a significant reduction in blood pressure in patients who have drug-resistant hypertension and had a systolic blood pressure above 160 mmHg prior to receiving the device. After four years of treatment, Rheos reduced systolic blood pressure by an average of 53mmHg (193 mmHg vs. 140 mmHg). Blood pressure was reduced significantly each year, with the largest decrease occurring in year four. Over this time period the number of medications that patients were taking to treat their hypertension decreased from an average of five at baseline to 3.4 medications at four years.
The Rheos System uses baroreflex activation therapy (BAT) that is designed to trigger the body’s own natural blood flow regulation system to treat high blood pressure and heart failure. The Rheos System works by electrically activating the baroreceptors, the body’s natural blood pressure sensors that regulate cardiovascular function. These baroreceptors are located on the carotid artery. When activated by the Rheos System, signals are sent through neural pathways to the brain, which responds by telling the:
• Arteries to relax, making it easier for blood to flow through the body and reducing cardiac exertion;
• Heart to slow down, allowing more time for the heart to fill with blood; and
• Kidneys to reduce fluid in the body, lowering both excessive blood pressure and workload on the heart.
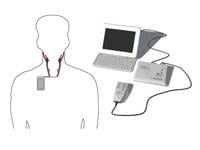
This system includes three components: a small device that is implanted under the collarbone, two thin lead wires that are implanted at the left and right carotid arteries and connected to the device, and the Rheos Programmer System, an external device used by doctors to noninvasively regulate the activation energy therapy from the device to the leads.
The therapy can be adjusted to meet each patient’s individual needs as they change over time, providing personalized treatment. The Rheos System is CE Marked and approved for sale for hypertension patients in Europe.


 January 05, 2026
January 05, 2026 









