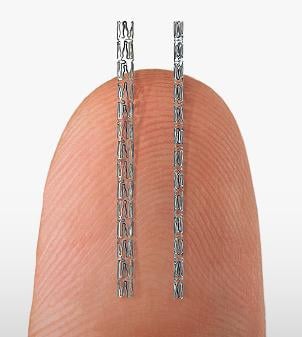
September 25, 2014 — Svelte Medical Systems reported that its drug-eluting coronary stent Integrated Delivery System (IDS) for percutaneous coronary intervention (PCI) met all DIRECT II study six-month angiographic and clinical endpoints. The IDS also exhibited reduced procedure and device times, with trends toward reduced fluoroscopy time and contrast use, confirming results seen in prior studies in which the IDS demonstrated procedural time and cost savings.
DIRECT II is a prospective, randomized, multi-center clinical study comparing the safety and efficacy of the Svelte drug-eluting coronary stent IDS and the Medtronic Resolute Integrity drug-eluting coronary stent in 159 patients undertaken in support of CE mark certification of the Svelte system. Non-inferiority to Resolute Integrity in the primary efficacy endpoint of in-stent late lumen loss (LLL) at six-months was clearly established (0.09+0.31 mm with Svelte IDS vs. 0.13+0.27 mm with Resolute Integrity, p-value for non-inferiority =<0.0001). Clinical outcomes were similarly positive, with six-month target lesion revascularization (TLR), target lesion failure, target vessel failure, myocardial infarction and major adverse cardiac event (MACE) rates in the Svelte IDS arm half those observed in the Resolute Integrity arm. Six-month TLR with the Svelte IDS was 0.9 percent, affirming results seen in the DIRECT first-in-man study in which zero TLR and MACE are now sustained through 28-months.
“The results of the DIRECT II study confirm the safety and effectiveness of this interesting new concept for coronary artery stenting,” said Stefan Verheye, M.D., Ph.D. and co-director of the Antwerp Cardiovascular Institute at the Middelheim Hospital in Antwerp. “The Svelte IDS can be delivered through smaller catheters and facilitates radial artery access, two increasingly important approaches to PCI demonstrating improved procedural outcomes and greater patient comfort.”
Svelte says its device represents a change in coronary stent delivery. Utilizing an integrated wire design, which provides the lowest crimped stent profile on the market, the IDS is designed to optimize trans-radial interventions (TRI) and a ‘slender’ approach to PCI by downsizing catheter sizes used during intervention. TRI is used in the majority of PCI in Japan and parts of Europe, and its use in the United States is growing rapidly, increasing from less than 5 percent of procedures in 2007 to 25 percent today. In the DIRECT II study, 67 percent of patients were treated via TRI.
“We thank the DIRECT II investigators, many of them first-time users of the IDS, for their contribution to this study which confirms the safety and efficacy of our system,” said Jack Darby, president and CEO of Svelte Medical Systems. “Achieving this important milestone brings us closer to offering clinicians and patients an entirely new approach to PCI which compliments TRI and slender stenting while simplifying the treatment of challenging lesions and extracting hard and soft procedural costs.”
Data from the DIRECT II study were presented during the 2014 Transcatheter Cardiovascular Therapeutics (TCT) conference. Both the IDS and a conventional rapid-exchange (RX) platform incorporating Balloon Control Band (BCB) technology will be offered with the Svelte drug-eluting stent and are expected to be commercially available in Europe in 2015.
For more information: www.sveltemedical.com


 July 02, 2024
July 02, 2024 









