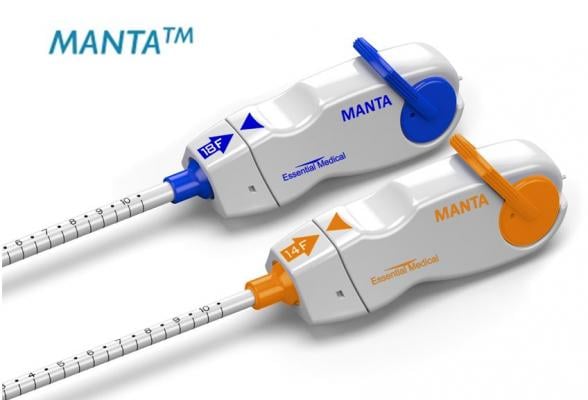
October 5, 2018 — Teleflex Inc. announced the acquisition of Essential Medical Inc. Based in Exton, Pa., Essential Medical is a privately-held medical device company that has developed and commercialized the CE Marked Manta Vascular Closure Device specifically designed for closure of large bore arteriotomies following procedures utilizing devices or sheaths ranging in size from 10F to 18F (with maximum outer diameters up to 25F).
In its CE Mark study, the Manta Device demonstrated rapid and reliable hemostasis with its resorbable collagen-based technology and complication rates that were non-inferior to surgical and suture-based closure methods.(1)
Teleflex President and CEO Liam Kelly said the acquisition expands his company’s presence in the structural heart and endovascular aneurysm repair markets. He added that the Manta device has been used in more than 8,100 procedures to date across a number of countries in the European Union (EU).
Kelly said U.S. Food and Drug Administration (FDA) premarket approval of the Manta Vascular Closure Device is anticipated for 2019. The MANTA Device is not currently approved for sale or distribution in the United States.
For more information: www.teleflex.com
Reference
(1) "Percutaneous Plug-Based Arteriotomy Closure Device for Large-Bore Access" by Nicolas M. Van Mieghem, MD, et al in Journal of the American College of Cardiology: Cardiovascular Interventions, Vol 10, No. 6, 2017.


 November 14, 2025
November 14, 2025 









