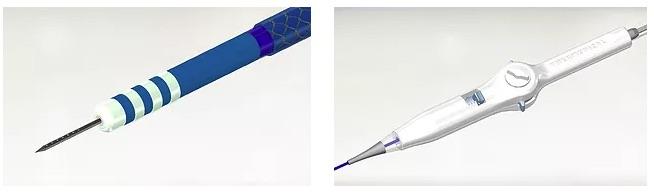
August 2, 2018 — Thermedical announced that the U.S. Food and Drug Administration (FDA) has approved its Investigational Device Exemption (IDE) application for the Early Feasibility Study (EFS) of the company’s Durablate ablation catheter. The single-arm, observational study is designed to evaluate the safety and effectiveness of the Durablate catheter to treat ventricular tachycardia (VT), a leading cause of sudden cardiac death in the United States.
The target population consists of patients who have already been treated with medicine, have an implantable cardioverter defibrillator (ICD), and who have had a conventional ablation procedure to treat VT, yet all their treatments have failed. The study is scheduled to begin in 2018 at Mayo Clinic under the direction of Douglas L. Packer, M.D., professor of medicine, and at Loyola University of Chicago Stritch School of Medicine, under the direction of David Wilber, M.D., medical director of clinical electrophysiology.
Implantable cardioverter defibrillators (ICDs) are the current standard-of-care treatment for patients suffering from VT; however, this approach does not stop the progression of the disease or provide a cure. In addition, ICDs can be costly and painful, and may substantially reduce a patient’s quality of life. VT patients who have ICDs and recurrent VT may be treated today with conventional RF ablation, which is a lengthy procedure that has a success rate of approximately 50 percent.
Thermedical’s patented ablation technology uses heat to kill the damaged heart muscle cells that are causing VT. The heat is delivered by the Durablate catheter, which simultaneously injects a precise flow of hot saline together with controlled RF energy into the heart tissue. This combination is an advanced form of biological heat transport that is 20 times more effective than conventional RF ablation methods. Compared to conventional VT ablation catheters, Durablate can more accurately control the ablation size, and it can treat tissue that is deeper in the heart wall, which is where life-threatening arrhythmias (abnormal, rapid heart rhythm) that cause VT are often located.
For more information: www.thermedical.com


 February 06, 2026
February 06, 2026 









