June 23, 2009 - Kensey Nash Corp. today said the ThromCat XT Thrombectomy Catheter System was granted CE marking approval, allowing for the sale of the product throughout the European Economic Area (EEA).
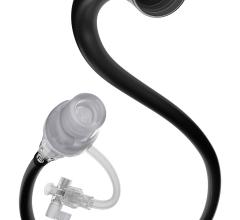
The ThromCat XT System, a mechanical thrombectomy catheter designed to remove thrombus from a patient, is indicated for use in both the coronary and peripheral vasculature. The CE mark approval meets the criteria established for a milestone payment of $1.5 million under the company's development and regulatory services agreement with The Spectranetics Corp. The milestone payment to Kensey Nash will be recorded as deferred revenue and will be recognized into revenue over the remaining period of the agreement.
The ThromCat XT System is based upon the same design principles as the original ThromCat product, which utilizes a high-speed rotary helix within a flexible atraumatic 7 Fr. extraction catheter to safely and effectively remove thrombus. The ThromCat XT extends the capability of this innovative first generation disposable device by incorporating an advanced extraction helix technology that improves the product's performance, particularly when used in more advanced thrombus. The ease-of-use of the ThromCat XT System is enhanced by further simplifying the setup procedure of the original device, resulting in an even shorter set up time. In addition, the device incorporates a new catheter jacket technology to allow use in more challenging anatomy, thereby extending the utility of this powerful and disposable device, the company said.
For more information: www.kenseynash.com


 January 19, 2026
January 19, 2026