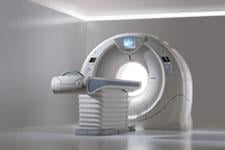
September 20, 2011 — Toshiba America Medical Systems Inc. will highlight its Aquilion One computed tomography (CT) system at the American Society of Emergency Radiology (ASER) conference, Sept. 14-17, 2011 in Miami. The company will specifically showcase the system’s application in an emergency department (ED) setting.
The Aquilion One can capture the entire heart or brain in one rotation and covers up to 16 cm of anatomy using 320 ultra-high-resolution 0.5 mm detector elements. It can also show an organ’s dynamic blood flow and real-time function. With the 160-detector row ultra helical scan mode, the entire chest, abdomen and pelvis can be imaged in fewer than five seconds; this is 2.5 times faster than 64-detector row imaging.
The ultra helical scanning mode produces significantly fewer motion artifacts and provides clinicians with high-quality images for accurate diagnoses.
The system also features numerous technologies that limit radiation dose to make CT imaging safer for patients. Adaptive Iterative Dose Reduction (AIDR) software improves the image by removing noise until the optimal image is produced. Additional dose reduction technologies include Target CTA, SUREExposure, SUREExposure Pediatric, QDS and Boost3D.
Also at ASER, Erin Angel, Ph.D., clinical sciences manager, CT, Toshiba America Medical Systems, will conduct a breakfast presentation titled “Learn How Aquilion CT Technology Helps to Manage Radiation Dose in Your Emergency Department.” The presentation will be held on Sept. 15, 7:15 a.m.–7:45 a.m., at the Ritz-Carlton, Key Biscayne, Fla.
For more information: www.medical.toshiba.com


 February 02, 2026
February 02, 2026 









