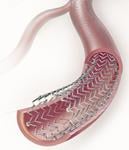
November 24, 2010 – Results for a trial comparing a drug-eluting stent to balloon angioplasty and bare metal stenting in treating restenosis after angioplasty were announced at the VEITHsymposium. The Zilver PTX drug-eluting stent met the trial's 12-month primary endpoints.
The stent has CE mark approval in Europe for the superficial femoral artery.
Michael Dake, professor of cardiothoracic surgery, Stanford University School of Medicine, said that 479 patients were enrolled at 56 institutions in the United States, Japan and Germany. Of those, 241 patients were randomized to the Zilver PTX group and 238 to the percutaneous transluminal angioplasty (PTA) group. Demographics and lesion characteristics were similar for the groups. Approximately half the PTA group experienced acute failure and underwent secondary randomization, assigning 59 and 61 patients to provisional stenting with Zilver BMS and Zilver PTX, respectively.
"The study results met the 12-month primary endpoint goals showing non-inferior EFS and superior patency for the Zilver PTX compared to PTA,” Dake said. “There was also significant clinical improvement with the Zilver PTX. Patients with de novo or artery re-narrowing, or restenosis, after angioplasty were randomized to PTA or Zilver PTX stent placement. PTA patients experiencing acute failure underwent secondary randomization to provisional stenting with Zilver BMS or Zilver PTX. Endpoints included event-free survival, stent integrity by radiographic core laboratory analysis, and primary patency by Duplex ultrasound core laboratory analysis.”
The results of this randomized, multicenter study support the safety and effectiveness of the stent.
For more information: www.VEITHpress.org


 January 05, 2026
January 05, 2026 









