December 29, 2017 — Vivasure Medical announced in October the successful enrollment of the first patient in the Frontier IV clinical study, a non-randomized multicenter international trial, designed to expand the indications of the PerQseal large arteriotomy closure technology. The patient was enrolled by Peter Crean, M.D., at the Blackrock Clinic, Dublin, Ireland.
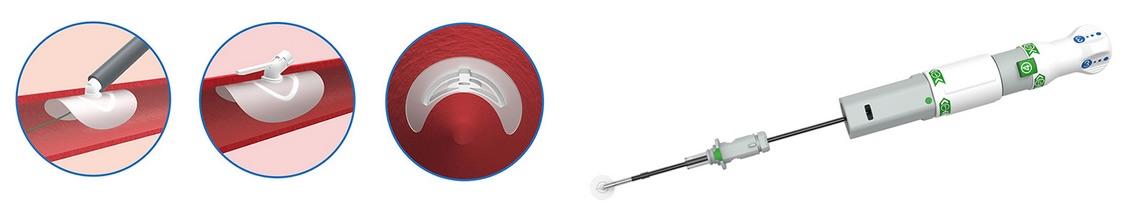
Large arteriotomies (12F+) are vessel punctures created to facilitate endovascular procedures such as transcatheter aortic valve replacement (TAVR), endovascular aneurysm repair (EVAR), balloon valvuloplasty (BAV) and ventricular assist device (VAD) implantation. PerQseal is the world’s first fully absorbable, patch-based large-bore percutaneous closure technology, according to Vivasure.
“Driven by clinical and economic outcomes data, percutaneous access-site management has become an increasingly important aspect of TAVR procedures,” said Christoph Naber, M.D., Ph.D., of the department of cardiology and angiology, Contilia Heart and Vascular Centre, Essen, Germany, and TAVR principal investigator of Frontier IV. “I strongly believe PerQseal, which is designed specifically to address large arteriotomies, will help improve outcomes for these patients.”
PerQseal utilizes a fully absorbable, intravascular patch, which seals large arteriotomies from the inside. It is comprised of a synthetic polymer implant and an easy-to-use, ergonomically designed delivery system. The implant has a flexible, low-profile intravascular patch and a supporting scaffold. A portion of the scaffold extends through the arteriotomy, and includes a locator which helps maintain the implant in position. After deployment, the implant is rapidly endothelialized and fully absorbed.
For more information: www.vivasuremedical.com
Related Content


 November 14, 2025
November 14, 2025 









