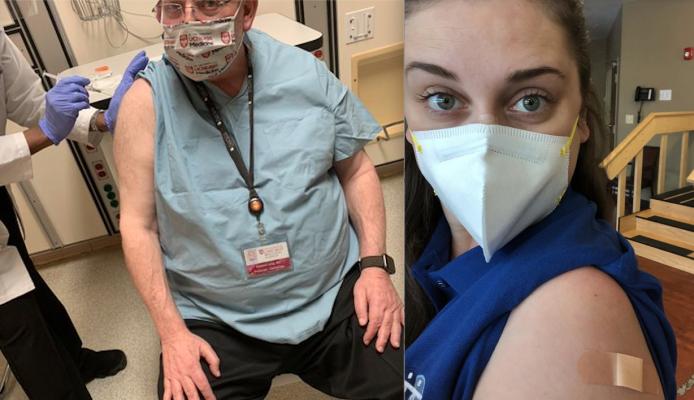
Roberto Lang, M.D., director of noninvasive cardiac imaging, University of Chicago Medical Center and former American Society of Echocardiography (ASE) president, received his first dose of the COVID vaccine in December. In addition to front line hospital workers, nursing home staff and residents also qualified for the first round of vaccinations. Right, Shannon Yaw, OTR/L, director of rehabilitation at a hard-hit nursing home in Michigan, received her first dose just before Christmas.
The Centers for Disease Control and Prevention (CDC) Dec. 3, released an interim guidance document to federal, state, and local jurisdictions on the allocation of initial doses of the COVID-19 vaccines. The document outlines who qualified for the first round of vaccinations with the Pfizer and Moderna vaccines. The priority order is given based on who is most at risk, those who are directly exposed to COVID carriers or patients on a daily bases and essential workers.
Phase 1a — Healthcare workers and Nursing Homes
1. Healthcare personnel
2. Residents of long-term care facilities (LTCFs)
Read the document The Advisory Committee on Immunization Practices’ Interim Recommendation for Allocating Initial Supplies of COVID-19 Vaccine — United States, 2020.
The report said about 21 million U.S. healthcare personnel work in settings such as hospitals, LTCFs, outpatient clinics, home healthcare, public health clinical services, emergency medical services and pharmacies. Healthcare personnel comprise clinical staff members, including nursing or medical assistants and support staff members (e.g., those who work in food, environmental and administrative services). Jurisdictions might consider first offering vaccine to healthcare personnel whose duties require proximity (within 6 feet) to other people. If vaccine supply remains constrained, additional factors might be considered for subprioritization.
About 3 million adults reside in LTCFs, which include skilled nursing facilities, nursing homes and assisted living facilities. Depending upon the number of initial vaccine doses available, jurisdictions might consider first offering vaccination to residents and healthcare personnel in skilled nursing facilities because of high medical acuity and COVID-19–associated mortality among residents in these settings.
Phase 1b — Police, Firefighters and Essential Workers
• Frontline essential workers such as fire fighters, police officers, corrections officers, food and agricultural workers, United States Postal Service workers, manufacturing workers, grocery store workers, public transit workers, and those who work in the educational sector, including teachers, support staff and daycare workers.
• People aged 75 years and older, because they are at high risk of hospitalization, illness, and death from COVID-19. People aged 75 years and older who are also residents of long-term care facilities should be offered vaccination in Phase 1a.
Phase 1c — People Aged 65 and Older, Other Essential Workers
• People aged 65-74 years, because they are at high risk of hospitalization, illness, and death from COVID-19. People aged 65-74 years who are also residents of long-term care facilities should be offered vaccination in Phase 1a.
• People aged 16-64 years with underlying medical conditions that increase the risk of serious, life-threatening complications from COVID-19.
• Other essential workers, such as people who work in transportation and logistics, food service, housing construction and finance, information technology, communications, energy, law, media, public safety, and public health.
Read more on the CDC vaccine prioritization plan.
Related COVID Content:
FDA Clears First COVID-19 Vaccine Under an Emergency Use Authorization
FDA Approved Second COVID-19 Vaccine From Moderna
FDA Clears Third COVID-19 Vaccine From Janssen
Cardiologists Should Prioritize Influenza Vaccination Amidst COVID-19 Pandemic
Who Qualifies for the COVID Vaccine Under CDC Guidelines
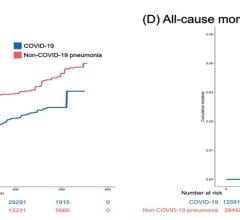
COVID-19 Patients With Cardiovascular Disease Have In-hospital Mortality Rates of 25 to 38 Percent
VIDEO: Lingering Myocardial Involvement After COVID-19 Infection — Interview with Aaron Baggish, M.D.
COVID-19 Can Impact Hearts in Young Children
COVID-19 Positive STEMI Patients Have Higher Mortality
VIDEO: ECMO Hemodynamic Support Effective in Sickest COVID-19 Patients — Interview with Ryan Barbaro, M.D.
The Cardiovascular Impact of COVID-19
COVID-19 Cardiovascular Registry Details Disparities Among Hospitalized Patients
VIDEO: How to Reduce COVID Exposure, Speed Exams and Cut Readmissions in Cardiology Departments— Interview with Keith Ellis, M.D.,

 March 20, 2024
March 20, 2024