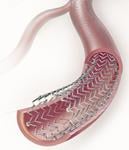
October 5, 2009 – In a breakthrough development that could dramatically reduce the number of leg amputations and painful bypass graft surgeries performed annually on European patients, a first-of-its kind drug-eluting stent for a widespread form of peripheral arterial disease (PAD) is now available to physicians throughout the European Union.
Cook Medical said its new CE-marked Zilver PTX Drug-Eluting Peripheral Stent is widely expected to improve the standard of care for many patients with serious blockages in the superficial femoral artery (SFA) by creating a highly effective, completely new treatment option. In the largest clinical trial of its kind ever conducted, the Zilver PTX stent was shown to effectively bridge the gap between the patient results achieved using open surgical bypass graft procedure, which is typically more painful and requires a longer hospital stay for the patient, and the less traumatic, but typically less effective, earlier minimally invasive treatment options for PAD such as balloon angioplasty and bare metal stenting[1].
Cook’s device was introduced commercially to European physicians at the annual CIRSE meeting, Sept. 19-23 in Lisbon. The launch includes several important developments, including the introduction of a new Web site, www.cookmedical.com/zilverptx, with clinical and practical information on the device for patients and physicians in English and major European language languages.
Cook also has initiated a first-of-its-kind open clinical database on SFA treatments. The SFA Open-Registry provides physicians with an opportunity to exchange ongoing, real world treatment results. Utilizing an intuitive Web interface, clinicians may track their patients, view the medical community’s trends in treating PAD and determine the most effective treatment options. Transparency will be a central objective of the SFA Open-Registry, with results for all treatment types available to participating physicians.
As part of its ongoing corporate mission to help reduce global healthcare delivery costs, Cook has adopted an “affordable innovation” strategy aimed at reducing any potential financial or reimbursement barriers to its widespread adoption as the standard of care for PAD in the SFA.
The first DES approved for treating PAD in the superficial femoral artery (SFA), the difficult-to-treat, largest blood vessel in the leg, the Zilver PTX stent first expands and holds open the artery to restore blood flow. The device then delivers the drug paclitaxel to the cells in the vessel wall to reduce the risk of new blockages forming. In a major advance over previous drug-eluting technologies, the Zilver PTX achieves targeted drug delivery without using a polymer to adhere the drug to the stent body. This eliminates the potential patient risks associated with polymer-coated devices, including clot formation and inflammation.
Cook licenses the rights to use paclitaxel on peripheral stents and other noncoronary medical devices from Angiotech Pharmaceuticals Inc. of Vancouver, British Columbia, Canada. In the United States, the Zilver PTX Drug-Eluting Stent is an investigational device not available for sale at this time.
For more information: www.cookmedical.com


 July 02, 2024
July 02, 2024 









