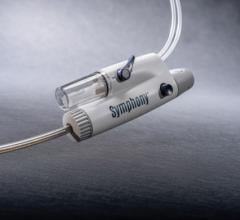
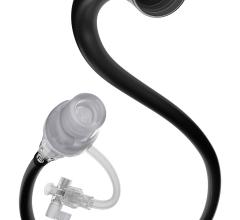
The new Fetch Aspiration Catheter from Possis Medical, Inc. has received 510(k) clearance from the FDA. Employing catheter technology from the company’s AngioJet Rheolytic Thrombectomy System, the Fetch catheter gives physicians another alternative for the aspiration of small, fresh blood clots (thrombus) and other embolic debris from arteries.
An industry-standard syringe is used as its aspiration source, and Fetch achieves flexibility and handling for interventional procedures through an advanced braided shaft design used in other AngioJet thrombectomy catheters marketed by Possis. Additionally, the Fetch catheter is 0.014” guidewire and 6F guide catheter compatible.


 January 19, 2026
January 19, 2026