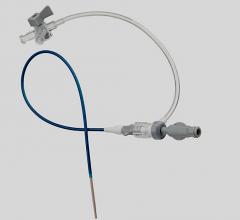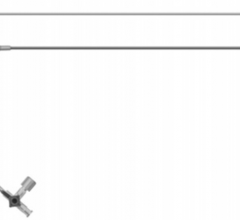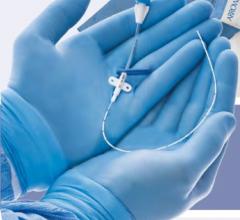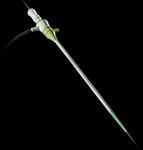
August 17, 2010 – Certain batches of the St. Jude Medical 6 French Engage Introducer are being recalled because the shaft of some devices may separate from the hub. The company is advising customers to discontinue use of the product.
The Engage Introducer is used to implant catheters and electrodes into blood vessels during interventional procedures and helps prevent blood loss. If the hub and shaft separated during a procedure, a life-threatening bleeding episode could occur.
St. Jude said the affected batches were made between April 27 and June 3, 2010, and include about 5,120 devices. The class I recall was initiated on June 28.
Affected products and batch numbers:
• Engage TR Introducer, 6 Fr. - ACT (2.25 mm), 7 cm length, .025" Max Guide wire, Batch: 3109782
• Engage TR Introducer, 6 Fr. - ACT (2.25 mm), 12 cm length, .025" Max Guide wire, Batch: 3105838
• Engage TR Introducer, 6 Fr. - ACT (2.25 mm), 25 cm length, .035" Max Guide wire, Batch: 3107645
• Engage TR Introducer, 6 Fr. - ACT (2.25 mm), 12 cm length, .035" Max Guide wire, Batches: 3103891, 3110889, 3110892, 3118794, 3123051
• Engage TR Introducer, 6 Fr. - ACT (2.25 mm), 12 cm length, .038" Max Guide wire, Batches: 3107789, 3107790
St. Jude Medical sent customers an urgent medical device recall notice letter dated June 24. The letter was addressed to cath lab managers and risk managers and described the problem and the products involved. Sales representatives were also sent a St. Jude Medical memorandum June 24 and a reconciliation form.
Class 1 recalls are the most serious type of recall and involve situations in which there is a reasonable probability that use of these products will cause serious adverse health consequences or death.
For more information: www.fda.gov

 June 10, 2020
June 10, 2020