Step 2/3
Related Content
Jan. 28, 2026 — Imperative Care has announced the commercial launch and first patient cases of the new Zoom 4S Catheter ...
Oct. 7, 2025 — Cagent Vascular has announced the commercial launch of the Serranator PTA Serration Balloon Catheter in 7 ...
Oct. 2, 2025 — AorticLab recently announced the U.S. Food and Drug Administration (FDA) has approved its Investigational ...
Sept. 29, 2025 — Radiation therapy may offer a comparable and potentially safer alternative to repeat catheter ablation ...
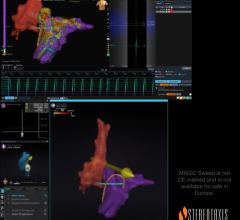
July 28, 2025 — Stereotaxis, a provider of surgical robotics for minimally invasive endovascular intervention, has ...
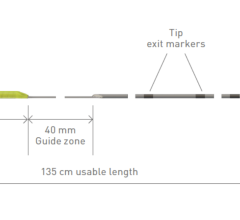
Sept. 16, 2024 — Biotronik has received U.S. Food and Drug Administration (FDA) labeling approval of its Selectra 3D ...
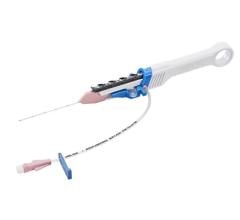
February 9, 2024 — BIOTRONIK introduced the Micro Rx catheter, a novel rapid exchange microcatheter developed to easily ...
January 4, 2024 — The days of prolonged fasting prior to cardiac catheterization may be numbered, as the body of ...
November 7, 2023 — Shockwave Medical, Inc, a pioneer in the development and commercialization of transformational ...
June 26, 2023 — The U.S. Food and Drug Administration (FDA) has issued a Class I recall on the Teleflex and Arrow ...


 January 29, 2026
January 29, 2026