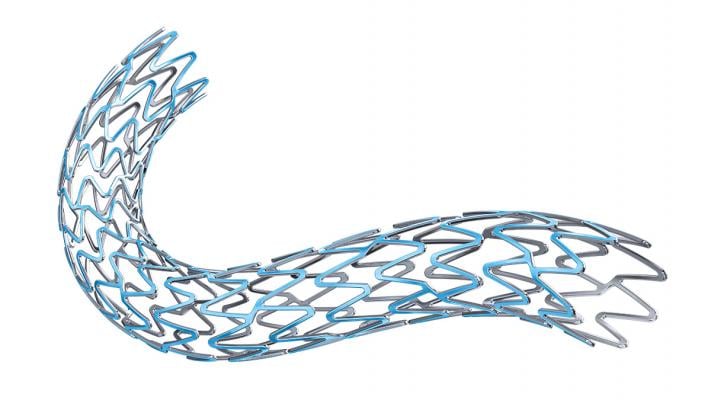
October 15, 2015 — Two new advances in stent technology announced in recent days further reinforce the effectiveness of the next-generation bioabsorbable stents. Both new stents are designed to help reduce the risk of long-term complications for patients receiving a stent and will give interventional cardiologists more options in the care of cardiovascular patients.
One-year results from the ABSORB III trial were presented at Transcatheter Cardiovascular Therapeutics (TCT) 2015 in San Francisco. The study met its primary endpoint, demonstrating the Absorb stent — which is fully absorbed by the body — performed as well as conventional stents at one year. Conducted at 193 sites, primarily in the United States, the ABSORB trial enrolled about 2,000 people with coronary artery disease.
The Society of Angiography and Cardiac Interventions (SCAI) issued a statement finding these initial data encouraging and that it looks forward to more long-term data over the next three to four years that will detail the benefits of this technology in participating patients. Although not yet commercially available in the United States, currently Absorb is available in more than 100 countries worldwide.
Last week, the U.S. Food and Drug Administration (FDA) approved Synergy, the first stent with a bioabsorbable polymer. Synergy’s drug coating and polymer are absorbed shortly after drug elution is complete at three months, leaving only the metal stent platform behind. This FDA approval is based on data from the EVOLVE II study, which showed Synergy performed as well as conventional stents at one year.
“These two new stents represent a new generation of stent technology. Both treat heart artery blockages like conventional stents, but are revolutionary in that they eliminate permanent stent components that occasionally cause long-term problems,” said SCAI Secretary Kirk Garratt, M.D., FSCAI. “These innovations highlight the interventional cardiology community’s continuous efforts to advance treatments that save lives and improve the quality of life for patients with cardiovascular disease.”
“Our goal is to ensure patients have the right treatment, at the right time,” said SCAI President James C. Blankenship, M.D., FSCAI. “Innovative technologies like bioabsorbable stents provide us with a wider array of options so we can offer patients the best possible treatment for their condition. We expect both stents will improve long-term outcomes for patients and reduce costs of treating coronary artery disease.”
For more information: www.scai.org


 November 14, 2025
November 14, 2025 









