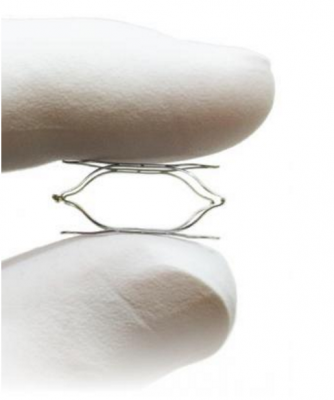
Aug. 15, 2017 — The U.S. Food and Drug Administration (FDA) has approved the Vascular Dynamics Inc. (VDI) investigational device exemption (IDE) study so it can initiate its pivotal trial for its MobiusHD System for the treatment of resistant hypertension.
The CALM (Controlling and Lowering Blood Pressure with MobiusHD) 2 trial is a multi-center, prospective, randomized, double-blind, sham-controlled pivotal study designed to evaluate the safety and effectiveness of the MobiusHD System. The company will evaluate patients from select locations throughout the United States whose hypertension remains uncontrolled despite using three or more anti-hypertensive pharmacologic therapies. VDI also intends to conduct the CALM 2 trial in certain European countries following appropriate regulatory authorization.
“The rigor and scientific focus of the Calm 2 trial has been crafted and improved from much of what we learned from prior studies. Calm 2 will offer patients a therapeutic alternative and a level of medical monitoring to which they otherwise would never have access outside of a clinical trial,” said Bryan Williams, co-principal investigator of the Calm 2 trial and chair of medicine at University College London, He is also director of the NIHR UCLH/UCL Biomedical Research Centre and director of research at UCL Hospitals. He is chairman-elect of the European Council on Hypertension of the European Society of Cardiology.
“In clinical practice, we are regularly faced with those hypertension patients who simply do not respond to medication or lifestyle changes,” said Gregg Stone, M.D., co-principal investigator of the CALM 2 trial and director of cardiovascular research and education for Columbia University Medical Center/New York-Presbyterian Hospital, and co-director of medical research and education at the Cardiovascular Research Foundation. “Initiating this trial is an important step toward identifying additional viable treatments to help this large population of patients.”
The company has been chosen as a participant in the FDA’s Expedited Access Pathway (EAP) program, a focused initiative to significantly accelerate access for US patients and their physicians to innovative medical treatments. VDI is also one of only nine companies chosen for the FDA’s Early Feasibility Study Investigational Device Exemptions (IDE) Pilot Program, which enables companies to conduct smaller-scale studies under the guidance of the agency in the United States in order to meet the requirements for an earlier pathway toward approval.
The MobiusHD System, a minimally-invasive system, capitalizes on the ability of the body’s baroreceptor mechanism to regulate blood pressure. Baroreceptors are receptors located in the carotid artery that sense blood pressure and relay that information to the brain. The MobiusHD implant is designed to amplify the signals received by the surrounding arterial baroreceptors, and thereby increase the body’s natural response to lower blood pressure through vasodilation.
The MobiusHDsystem has received a CE mark for the treatment of hypertension in the European Union. The MobiusHD system is not commercially available in the United States.
About Resistant Hypertension
Hypertension, or elevated blood pressure, is a common medical condition that currently affects one billion people worldwide.[1] If left untreated, hypertension can cause life-threatening problems, including heart attack, aneurysm, stroke or kidney failure. Patients with hypertension can often reduce their risk factors by making lifestyle changes such as losing weight, quitting smoking, and increased exercise. In cases with advanced hypertension, medical therapies may be prescribed.
Resistant hypertension cannot be controlled with medical therapies. Patients experiencing resistant hypertension are at four times greater risk of cardiovascular events compared with hypertensive patients achieving blood pressure targets.[2] The American Heart Association (AHA) estimates that high blood pressure costs the U.S. $46 billion each year, including the cost of healthcare services, medications to treat high blood pressure and lost productivity.
For more information: www.vasculardynamics.com
Watch the VIDEO “Device Therapies to Treat Hypertension,” an interview with Krishna Rocha-Singh, M.D., Prairie Vascular Institute, Springfield, Ill., explains advancements in device therapy for hypertension at the Transcatheter Cardiovascular Therapeutics (TCT) 2016 annual meeting.
References:


 November 14, 2025
November 14, 2025 









