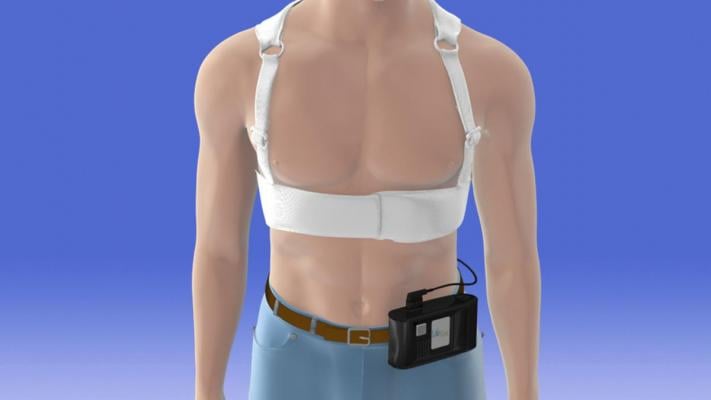
June 27, 2018 — Wearable cardioverter defibrillators may be a safe and effective alternative to surgically implanted devices in children with ventricular heart rhythm disorders that put them at risk for sudden cardiac death, according to new research. The vest-like devices that deliver electric shocks to interrupt a dangerous heart rhythm are discussed in a new study published in Circulation: Arrhythmia and Electrophysiology, an American Heart Association journal.
“Our results, which stem from the largest study to date among children in the United States using wearable cardioverter defibrillators, suggest that these external devices can help save the lives of children who are at the time, not good candidates for surgically implanted defibrillators because of their medical condition,” said the study’s principal investigator David Spar, M.D., assistant professor of pediatrics at the University of Cincinnati and a pediatric electrophysiologist at Cincinnati Children’s Hospital.
Sudden cardiac arrests in children are usually caused by heart abnormalities that the child is born with. Cardiac arrests are often triggered by an electrical malfunction that results in a rapid heartbeat that causes the heart to pump ineffectively. With its pumping action disrupted, the heart cannot pump blood to the brain, lungs and other organs, and death can occur if the heart’s rhythm is not restored or corrected.
Surgically implanted cardioverter defibrillators — the main therapy for children at high-risk for sudden cardiac death due to heart rhythm disorders, known as arrythmias — are effective in averting deaths from potentially lethal heart rhythms, but invasive surgery or prolonged hospitalization is required.
However, many young patients are not good candidates for these surgically implanted devices because they need only a temporary “bridge” to help their heart. For example, if they are waiting for a heart transplant, are a newly diagnosed heart failure patient who is recovering cardiac function or if they have an infected implanted cardioverter defibrillator, they would not be good candidates, Spar explained.
Even though wearable cardioverter defibrillators were approved by the U.S. Food and Drug Administration (FDA) for use in pediatric patients in 2015, data about their safety and efficacy in children has remained limited. This gap in knowledge has been particularly troublesome in how effective the device would be in younger patients in treating dangerous arrhythmias. If effective, the wearable cardioverter defibrillator could avoid prolonged hospitalizations while still offering arrhythmia protection, researchers said.
The newly published results -- based on a review of clinical outcomes among all U.S. pediatric patients who wore wearable cardioverter defibrillators between 2009 and 2016 — is the first to describe appropriate therapy with a wearable cardioverter defibrillator in a pediatric population.
Of the 455 patients (average age 15) included in the analysis, eight (1.8 percent) received at least one shock treatment to interrupt a dangerous heart rhythm. Six of the eight patients in whom the device discharged received appropriate therapy for the type of heart rhythm the device is designed to detect and stop.
In the two cases of inappropriate therapy, the device misfired when it misread a signal from the patient’s heart. In all cases, the dangerous heart rhythm was successfully interrupted, normal heart rhythm restored and the patient survived.
Co-authors on the study were Nicole Bianco, Ph.D.; Timothy Knilans, M.D.; Richard, Czosek, M.D.;and Jeffrey Anderson, M.D. Disclosures are listed on the manuscript
This study did not receive outside funding.
For more information: www.circep.ahajournals.org
Reference


 January 05, 2026
January 05, 2026 









