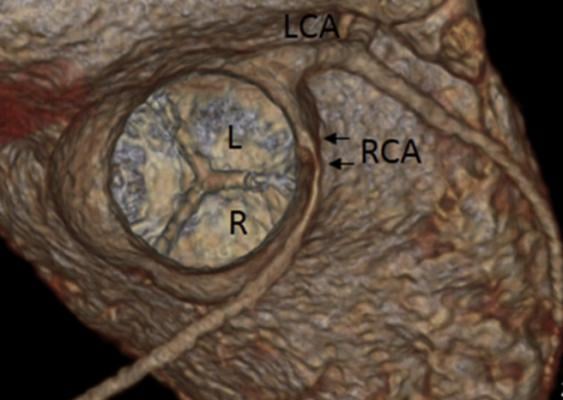
A 3-D CT reconstruction of an anomalous right coronary artery (RCA) can be seen arising from the left sinus of valsalva in a short-axis equivalent view. This is one of many images included in the new ASE guidelines to illustrate anatomical coronary artery variations.
March 5, 2020 — Experts in the medical imaging community have developed a new, landmark consensus document to optimize care of patients with congenital coronary anomalies. These defects of the blood vessels that supply blood to the heart muscles can be an important cause of a heart attack and sudden cardiac death in children and young adults, but historically they have been difficult to identify without cardiac catheterization. However, recent advances in multimodality imaging techniques have demonstrated increasing utility in the characterization of most congenital coronary anomalies in all age groups, and these techniques can complement or reduce the need for invasive angiography in many cases.
The new document, "Recommendations for Multimodality Assessment of Congenital Coronary Anomalies: A Guide from the American Society of Echocardiography (ASE)" provides guidelines for optimization of imaging for congenital coronary anomalies. [1] It includes a review of the benefits and limitations of the different imaging techniques, including echocardiography, cardiac computed tomography, cardiac magnetic resonance imaging, nuclear myocardial perfusion imaging and angiography. This guideline was developed in collaboration with the Society for Cardiovascular Angiography and Interventions (SCAI), the Japanese Society of Echocardiography, and the Society for Cardiovascular Magnetic Resonance (SCMR), and has also been endorsed by 17 ASE international alliance partners.
“Congenital coronary artery anomalies, both in isolation and associated with other forms of congenital heart disease, have been recognized as having significant potential morbidity and mortality, including sudden cardiac death in children and adolescents. This document outlines specific strategies for imaging of each of the known congenital coronary anomalies, providing cardiac imaging specialists with a valuable resource to improve patient care and foster responsible utilization of diagnostic imaging modalities," Explained ASE’s Chair of the writing group, Peter Frommelt, M.D., FASE, of Children’s Hospital of Wisconsin and the Medical College of Wisconsin in Milwaukee.
In conjunction with the publication of this guideline, Frommelt will conduct a live webinar, including a question and answer section, Tuesday, April 28, 2020, at 1 p.m. Eastern time. The webinar will be available for free to all ASE members and open to all other clinicians for $25. Registration and access to all ASE-hosted guideline webinars is available on ASEUniversity.org.
The full guideline document is available on the Journal of American Society of Echocardiography (JASE) website (OnlineJASE.com). This document and all ASE guideline documents are also available to the medical community at ASEcho.org/Guidelines.
Reference:

 February 06, 2026
February 06, 2026 









