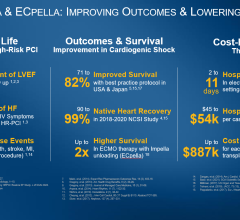
August 3, 2020 — A three-year, investigator-led, prospective study of Japanese patients who received an Impella heart pump finds use of Impella is associated with a 77 percent survival rate at 30 days in acute myocardial infarction (AMI) cardiogenic shock patients. The historic survival rate for cardiogenic shock is approximately 50 percent.
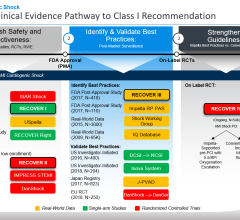
 The study, conducted with oversight by 10 Japanese professional societies, including the Japanese Circulation Society (JCS), is the first to use the Japanese Registry for Percutaneous Ventricular Assist Devices (J-PVAD) to investigate the efficacy and safety of Impella. J-PVAD data is independently monitored and shared with the Japan Pharmaceuticals and Medical Devices Agency (PMDA). Lead investigator Yoshiki Sawa M.D., Ph.D., presented the interim analysis as a late-breaking clinical study at the annual scientific meeting of JCS.
The study, conducted with oversight by 10 Japanese professional societies, including the Japanese Circulation Society (JCS), is the first to use the Japanese Registry for Percutaneous Ventricular Assist Devices (J-PVAD) to investigate the efficacy and safety of Impella. J-PVAD data is independently monitored and shared with the Japan Pharmaceuticals and Medical Devices Agency (PMDA). Lead investigator Yoshiki Sawa M.D., Ph.D., presented the interim analysis as a late-breaking clinical study at the annual scientific meeting of JCS.
“This was accomplished by closely following best practices for Impella use," Sawa said. "I thank my colleagues in the Japanese clinical community for following protocols and guidance from the Impella Committee to safely introduce a new therapy for the benefit of our patients.”
The interim analysis examined 819 patients treated with Impella for a variety of conditions, including cardiogenic shock and fulminant myocarditis, at 109 hospitals in Japan. Other findings include that Impella therapy is a highly effective treatment for fulminant myocarditis, with an 88 percent survival rate at 30 days. Overall, investigators conclude the favorable 30-day survival data indicates Impella is a beneficial therapy.
“This data demonstrates early use of Impella in drug-resistant acute heart failure patients can consistently achieve high rates of survival and native heart recovery for those in cardiogenic shock and other serious life-threatening conditions,” said Sawa, a professor at the Department of Cardiovascular Surgery at Osaka University Graduate School of Medicine and president of the Japanese Association of Thoracic Surgery. “This was accomplished by closely following best practices for Impella use. I thank my colleagues in the Japanese clinical community for following protocols and guidance from the Impella Committee to safely introduce a new therapy for the benefit of our patients.”
A previous analysis of cardiogenic shock survival rates in Japan by Ueki et al. (Circulation, 2015), found a 32% survival rate at 30 days in patients who received venous-arterial extracorporeal membrane oxygenation (V-A ECMO). The comparatively high survival rates with Impella were achieved by following established best practices that include placing Impella pre-PCI, identifying cardiogenic shock early, using a right heart catheter, and reducing use of inotropes. The study’s findings about the use of best practices are consistent with other published investigator-led studies, such as the National Cardiogenic Shock Initiative Study (NCSI) and the Inova study by Tehrani et al., that have demonstrated significant increases in survival with the use of Impella best practice protocols.
“This encouraging study demonstrates, when known best practices are followed, significant improvements in cardiogenic shock survival rates can be achieved with use of Impella,” said William O’Neill, M.D., medical director of the Center for Structural Heart Disease at Henry Ford Hospital and principal investigator of the NCSI Study. “I encourage physicians around the world to take note of the Japanese data, which demonstrates the rationale for early use of Impella in cardiogenic shock patients.
In an online video, Sawa and Abiomed’s chief medical officer, Chuck Simonton, M.D., discuss how the use of Impella and best practices helped Japanese physicians achieve high survival rates. Watch the video.
Native heart recovery with Impella is a cost-effective therapy of particular importance in Japan, a country with a limited number of heart transplants. The J-PVAD registry study is overseen by the Council for Clinical Use of Ventricular Assist Device Related Academic Societies (Japan VAD Council), Impella Committee, which is comprised of 10 organizations:
• The Japanese Circulation Society (JCS)
• Japanese Association of Cardiovascular Intervention and Therapeutics (CVIT)
• Japanese College of Cardiology (JCC)
• The Japanese Heart Failure Society (JHFS)
• Japanese Society for Artificial Organs (JSAO)
• The Japanese Society of Intensive Care Medicine (JSICM)
• Japanese Society of Pediatric Cardiology and Cardiac Surgery (JSPCCS)
• The Japanese Association for Thoracic Surgery (JATS)
• The Japanese Society for Cardiovascular Surgery (JSCVS)
• Japanese Society of Percutaneous Cardiopulmonary Support / Extracorporeal Membrane Oxygenation (PCPS/ECMO)
The Impella heart pump is manufactured by Abiomed. It has more than 14 years of FDA studies, real-world clinical data on more than 125,000 patients, and more than 650 peer-reviewed publications.
For more information: www.impella.com


 April 28, 2023
April 28, 2023