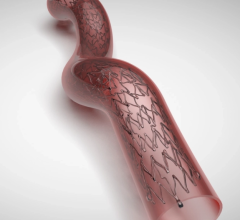
October 20, 2021 — Boston Scientific Corporation announced positive data for the Eluvia Drug-Eluting Vascular Stent System (Eluvia stent) during a late-breaking clinical trial presentation at the Vascular InterVentional Advances (VIVA) meeting in Las Vegas. Data presented included one-year results from the EMINENT trial, which demonstrated superiority of the Eluvia stent compared to self-expanding bare metal stents (BMS) for the treatment of patients with peripheral artery disease (PAD) and superficial femoral artery (SFA) or popliteal artery (PPA) lesions up to 210 mm in length. The study enrolled 775 patients, making it the largest randomized trial of a drug-eluting stent for the treatment of PAD to date.
In the trial, the Eluvia stent exhibited superiority with a primary patency rate of 85.4% versus 76.3% with BMS (p=0.0077).1 The analysis also confirmed a significantly greater rate of sustained clinical improvement without reintervention, 83.0% for patients treated with the Eluvia stent compared to 76.6% for those treated with BMS (p=0.0450). Further, there was no significant difference in major adverse events or all-cause mortality rates between patients treated with the Eluvia stent and those treated with BMS through one year.
"I am honored to have been part of this global study, which adds to the robust body of evidence from the IMPERIAL trial and confirms that the Eluvia stent should be considered the stent of choice for treating SFA and PPA lesions of intermediate length," said Professor Yann Gouëffic, M.D., Department of Vascular and Endovascular Surgery at Paris Saint-Joseph Hospital, France and principal investigator of the EMINENT study. "The superior primary patency rates and greater rates of clinical improvement without reintervention are reassuring for physicians looking to make clinically-based treatment decisions for their patients and reduce the need for repeat procedures."
The Eluvia stent was developed for the treatment of PAD – the narrowing of the arteries of the legs due to plaque buildup – which affects approximately 8.5 million people in the United States and more than 200 million people worldwide. Left untreated, PAD restricts blood flow to the legs and feet and patients often experience pain, swelling and a diminished quality of life. The Eluvia stent, which features sustained release of the lowest dose of paclitaxel of any peripheral drug-eluting device, re-opens blocked arteries and restores blood flow while utilizing a drug-polymer combination to prevent tissue regrowth.
"We are committed to meaningful clinical trials designed to evolve clinical practice, and on the heels of the positive RANGER II SFA data presented yesterday, we are pleased that the EMINENT trial establishes the Eluvia stent as the first drug-eluting stent to demonstrate superior primary patency rates compared to bare metal stents in a head-to-head randomized trial," said Michael R. Jaff, D.O., chief medical officer and vice president clinical affairs, technology and innovation, Peripheral Interventions, Boston Scientific. "The breadth of our portfolio, as the only company offering both a drug-coated balloon and a drug-eluting stent for the treatment of patients with PAD, provides physicians with evidence-based and highly-differentiated treatment options."
For more information: www.bostonscientific.com
Related Peripheral Stent Content:
New Peripheral Stent Technology
Boston Scientific Receives FDA Approval for Eluvia Drug-Eluting Vascular Stent
More news on Peripheral Artery Disease (PAD) technologies
CMS Grants Additional Reimbursement for Eluvia Vascular Stent


 November 09, 2025
November 09, 2025