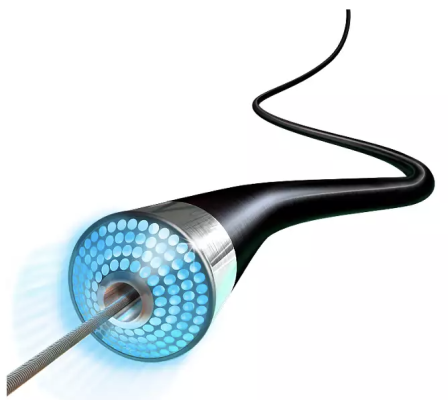
Northeast Scientific Inc., pioneers in reprocessing single use peripheral vascular catheters, announced this week it has received FDA clearance for reprocessing the Philips Spectranetics 0.9mm OTW Turbo-Elite laser atherectomy catheter.
Getty Images
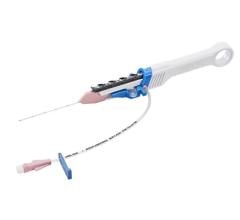
July 28, 2022 — Northeast Scientific Inc., pioneers in reprocessing single use peripheral vascular catheters, announced this week it has received FDA clearance for reprocessing the Philips Spectranetics 0.9mm OTW Turbo-Elite laser atherectomy catheter. This is yet another first of its kind accomplishment for the Connecticut based company, as this is the first time the FDA has awarded a 510(k) clearance for reprocessing this type of atherectomy catheter.
The device is used to treat peripheral arterial disease (PAD) and emits high energy ultraviolet light to vaporize blockages inside the vessels. This laser catheter is one of the most used modalities for this common procedure with treatments done in acute care settings, surgical centers as well as in the Office Based Lab (OBL).
CEO and Founder, Craig Allmendinger discussed what this means to the company by saying, “It’s validating to receive this clearance from the FDA after all the work our team undertook to meet the standards of earning a 510(k). The team at the FDA pushed us hard, and rightfully so, to prove that our methods and processes created an equivalent device to the original after reprocessing.”
He went on to say, “There is no doubt now that what we are doing is helping redefine the medical device landscape as we add another device type to our reprocessing portfolio. As we continue to expand our device types and reach, we look to further help the OBL Physician and never compromise on patient safety.”
Director of Product Development, Matt Farley had this to say; “We knew undertaking the Turbo-Elite device would be a difficult task as no-one had ever reprocessed a laser-based atherectomy device to date. Our team at all levels, from R&D to the product development team to the technicians helping do the work on the validations all have credit in making this product be cleared for reprocessing.”
The company has said that it will have an additional announcement soon regarding when the 0.9mm OTW Turbo-Elite laser atherectomy catheter device will be available for sale.
For more information: Smarthealth-care.com


 January 29, 2026
January 29, 2026