Getty Images
July 19, 2023 — The U.S. Food and Drug Administration (FDA) is recommending health care providers, laboratory personnel, and facilities stop using the Quidel Triage Cardiac Panel, manufactured by Quidel Cardiovascular Inc. (QuidelOrtho).
On May 25, 2023, QuidelOrtho issued an Urgent Product Correction Notification to customers in which they recalled certain lots of the Quidel Triage Cardiac Panel after receiving reports that tests provide inaccurate, lower than expected troponin levels in samples. On July 12, 2023, QuidelOrtho issued an updated Notification with additional recommendations to affected customers who did not confirm they have alternative testing methods available.
A falsely low or false negative troponin test result may lead to failure:
- to timely diagnose myocardial infarction (heart attack), particularly non-ST-segment elevation myocardial infarction (NSTEMI)
- to begin appropriate clinical intervention to prevent damage or injury to heart muscle
Currently, limited lots of unaffected Quidel Triage Cardiac Panel are available for distribution, and QuidelOrtho is prioritizing resupplying customers with no available testing alternatives. The FDA is recommending that health care providers, laboratory personnel, and facilities consider alternative products or alternative testing sites, as outlined in our recommendations below.
Recommendations
- The FDA recommends that health care providers, laboratory personnel, and facilities that currently use the Quidel Triage Cardiac Panel:
- Immediately stop using this product and use an alternate method.
- If no alternate method is available, consider sending patients to another local testing site in which an alternate method is available.
- If you do not have an alternate method and another local testing site with an alternate method is not available, follow QuidelOrtho’s May 25, 2023, Urgent Product Correction Notification:
- Flag ALL negative results reported to clinicians as possibly inaccurate until lots of unaffected product are available.
- Use results from an alternate clinical laboratory analyzer when troponin results are below or close to the cutoff and myocardial infarction is suspected.
- Perform serial sampling. Keep patients until at least three negative troponin values have been obtained.
- Use all Triage troponin results in conjunction with the patient’s risk factors, clinical presentation, EKG, and other imaging.
- Consider recommendations by the ACC, ESC guidelines and the Fourth Universal Definition of Myocardial Infarction for monitoring a patient for a rise or fall pattern of troponin.
Users without an alternate testing method or another local testing site with an alternative testing method may choose to continue using the affected product but should take the extra precautions provided above. If you are unable to switch to an alternative method and additional products are needed, QuidelOrtho has a small quantity of unaffected products to use as replacement.
Background
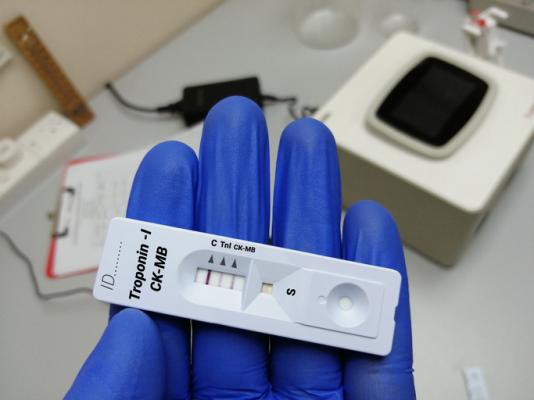
The Quidel Triage Cardiac Panel is a blood test that detects amounts of specific enzymes (creatine kinase MB or CK-MB) and proteins (myoglobin and troponin I) in blood or plasma samples. Use of these products could cause serious injury or death.
The Quidel Triage Cardiac Panel is a prescription use only device and it may be performed in certain laboratories and health care facilities including certain point-of-care or near patient settings.
Overall, the FDA does not anticipate a shortage as adequate alternate tests are available from alternative suppliers. However, in some limited instances, a facility may face a delay in procuring a new assay and certain facilities might have no alternatives to using the affected product.
FDA Actions
The FDA has identified this as a Class I recall, the most serious type of recall. The FDA is working with QuidelOrtho to further evaluate the issue and identify potential contributing factors.
The FDA will continue to keep health care providers, laboratory personnel, facilities, and the public informed if new or additional information becomes available.
Reporting Problems to the FDA
The FDA encourages users to report any adverse events or suspected adverse events experienced with the Quidel Triage Cardiac Panel.
- You can submit voluntary reports through MedWatch, the FDA Safety Information and Adverse Event Reporting program.
- Device manufacturers and user facilities must comply with the applicable Medical Device Reporting (MDR) regulations.
- Health care personnel employed by facilities that are subject to the FDA's user facility reporting requirements should follow the reporting procedures established by their facilities.
By promptly reporting adverse events, you can help the FDA identify and better understand the risks associated with medical devices.
Contact Information
If you have questions about this letter, contact the Division of Industry and Consumer Education (DICE).
Related Content:


 November 12, 2025
November 12, 2025 









