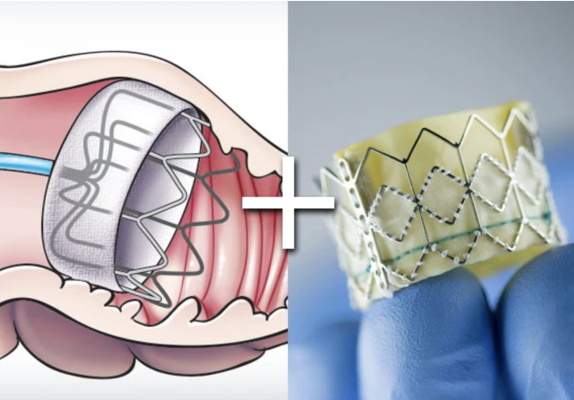
October 25, 2023 — Findings from a trial led by Cleveland Clinic show that patients with atrial fibrillation undergoing a transcatheter aortic valve replacement (TAVR) at the same time as a left atrial appendage occlusion (LAAO) procedure using the Watchman device had similar outcomes when compared to patients getting TAVR in addition to medical therapy or blood thinners.
Results from the “Safety and Efficacy of Left Atrial Appendage Occlusion at the Time of Transcatheter Aortic Valve Replacement — The WATCH TAVR Trial” were presented today during a late-breaking clinical trial session at the 2023 annual Transcatheter Cardiovascular Therapeutics conference and simultaneously published in the journal Circulation.
“WATCH TAVR is a novel study where two devices were used in order to combine the TAVR procedure for patient convenience and safety,” said lead author Samir Kapadia, M.D., chairman of Cardiovascular Medicine at Cleveland Clinic. “If patients can get both procedures done at the same time, that eliminates the need to be on blood thinners, which can have significant side effects. In addition, many patients can't take the medications due to high risk of bleeding.”
TAVR is most commonly the first-line treatment for patients over 65 years old with symptomatic severe aortic stenosis or narrowing of the aortic valve. Up to 40% of these patients experience atrial fibrillation, a type of abnormal heart rhythm. Atrial fibrillation also can increase the risk of stroke, and patients with atrial fibrillation undergoing TAVR have an increased risk of death, stroke and rehospitalization compared to those without atrial fibrillation.
The multicenter, randomized WATCH-TAVR trial set out to investigate the safety of implantation of the WATCHMAN 2.5device at the time of TAVR and to compare the efficacy of WATCHMAN LAAO with contemporary medical therapy in this population of patients with an increased risk of bleeding and stroke.
The trial enrolled 349 patients with severe aortic stenosis and atrial fibrillation. 177 patients received TAVR plus the LAAO procedure and 172 patients received the TAVR plus medical therapy. The trial took place between December 2017 and November 2020 at 34 centers in the United States. The mean age was 81 years old.
Patients in the TAVR plus medical therapy control group were managed by their treating physician, which allowed for the use of a direct oral anticoagulant, warfarin or no blood thinners, based on the physician’s discretion. Approximately one third of patients who were in the medical therapy control group were not on blood thinners at two years.
The primary endpoint, a collection of all-cause death, stroke and major bleeding at two years, showed that there were no differences in outcomes for patients when getting TAVR plus LAAO compared to getting TAVR plus medical therapy. However, there was a higher rate of blood clots, mainly more frequent blood clots in the veins, in patients undergoing TAVR plus LAAO compared to TAVR plus medical therapy.
In the trial, the WATCHMAN patients received blood thinners for 45 days followed by dual antiplatelet therapy until six months. Anticoagulation was based on the treating physician’s preference for patients randomized to TAVR plus medical therapy.
At baseline, 85.4% of patients were taking blood thinners and 71.3% of patients were on antiplatelet therapy. After 24 months, 82.5% compared to 50.8% of patients were on any antiplatelet therapy, and 13.9% compared to 66.7% of patients were on any blood thinner therapy in the TAVR plus LAAO group compared to TAVR plus medical therapy group, respectively.
“Although the findings showed similar outcomes, the increased complexity and risks of the combined procedure should be considered when LAAO is viewed as an alternative to medical therapy for patients with atrial fibrillation undergoing TAVR,” said Dr. Kapadia.
The trial was coordinated by the Cleveland Clinic Coordinating Center for Clinical Research and funded by a research grant from Boston Scientific. Dr. Kapadia has not been compensated by Boston Scientific for his work on the WATCH TAVR trial.
For more information: https://my.clevelandclinic.org/
Find more TCT23 conference coverage here
Related Content:
Real-world outcomes from the EU-PORIA registry of the FARAPULSE Pulsed Field Ablation (PFA) System
Late-Breaking Study Results Reinforce Positive Real-World Outcomes with the WATCHMAN FLX LAAC Device
PINNACLE FLX Study of New Watchman FLX LAA Occluder Meets Safety and Efficacy Endpoints
First Comparison of Amulet and Watchman FLX LAA Closure Devices Found Similar Outcomes
Pulsed Field Ablation Successfully Treats Atrial Fibrillation
Pulsed AF Trial Shows Pulsed Field Ablation May be Safer Than Tranditional RF Ablations


 October 31, 2025
October 31, 2025 









