Photo: Royal Philips
Dec. 15, 2025 — Royal Philips has entered into an agreement to acquire SpectraWAVE, Inc., an innovator in enhanced vascular imaging (EVI) of coronary arteries, angiography-based physiology assessments and the use of AI in medical imaging3. SpectraWAVE’s intravascular imaging and physiological assessment technologies provide solutions for treating patients with coronary artery disease, the most frequent type of heart disease, affecting 300+ million people worldwide4. SpectraWAVE is based in Bedford, Massachusetts, and currently employs more than 70 people.
“Our global leadership in image guided therapy is driven by deep clinical collaboration combined with our latest technology insights across hardware, software and AI, to innovate interventional procedures for better and more patient impact. Our world-class portfolio integrates interventional systems and devices into one platform, Azurion, serving patients worldwide.
“We are doubling down on image-guided therapy and expanding our portfolio in the coronary intervention segment with the addition of SpectraWAVE’s AI-powered innovations in high-definition intravascular imaging and angio-based physiological assessment, enabling us to deliver better care for more people,” said Roy Jakobs, CEO of Royal Philips.
Percutaneous coronary interventions are minimally invasive procedures that leverage intravascular imaging and physiological assessment to treat coronary artery disease. A significant and growing body of evidence shows that the use of intravascular imaging and physiological assessment technologies significantly improves patient outcomes for percutaneous coronary interventions5. With its Azurion image-guided therapy platform, integrated with its expanding portfolio of advanced diagnostic and treatment devices, Philips is driving the adoption of advanced healthcare technology to treat a growing and increasingly complex patient population.
“Philips shares our deep conviction that the convergence of intravascular imaging, coronary physiology and AI can fundamentally improve how every patient with coronary disease is treated. This partnership allows us to integrate and scale HyperVue and X1-FFR into the world’s leading image-guided therapy ecosystem, expanding choice for clinicians and supporting more consistent, high-quality care for the millions of patients who depend on coronary intervention each year,” said Eman Namati, PhD, CEO of SpectraWAVE.
“With today’s announcement we continue to expand the role of minimally invasive image guided therapy procedures, which are associated with better patient outcomes and improved cost-effectiveness 6,7. The acquisition of SpectraWAVE’s next-generation technologies for coronary intravascular imaging and physiological assessment mark a significant step in expanding our portfolio with breakthrough, AI-powered technologies that help clinicians decide, guide, treat and confirm treatment in one setting,” said Bert van Meurs, Chief Business Leader Image Guided Therapy at Philips.
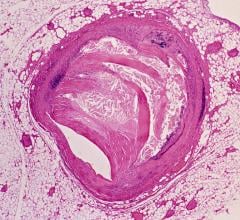
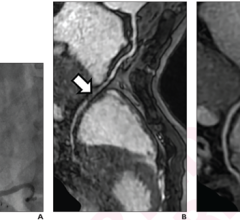
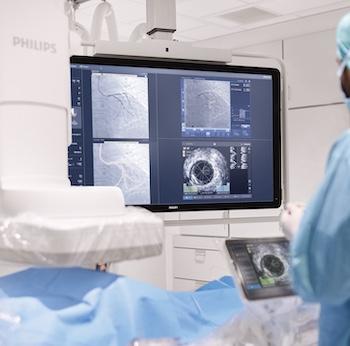
SpectraWAVE’s HyperVue Imaging System is an intravascular imaging platform that combines DeepOCT (next generation comprehensive optical coherence tomography) and NIRS (near-infrared spectroscopy) into the Enhanced Vascular Imaging (EVI) novel imaging segment to provide detailed structural and compositional images of the coronary arteries during percutaneous coronary interventions, with rapid setup, acquisition, and automated AI image analysis. Combined with Philips’ Eagle Eye Platinum digital IVUS and IntraSight technologies, HyperVue will expand clinicians’ intravascular imaging toolbox – IVUS, DeepOCT, NIRS, and wire and angio-derived physiology – all orchestrated through integrated systems to tailor guidance to each patient and lesion.
SpectraWAVE’s X1-FFR is an angiography-derived, AI-enabled physiology solution that calculates Fractional Flow Reserve (FFR) from a single coronary angiogram, providing a non-invasive ischemia assessment and turning routine X-ray images into coronary physiology data for simplified percutaneous coronary intervention workflows. X1-FFR complements Philips OmniWire iFR technology by extending physiologic guidance to wire-free scenarios and equipping clinicians with a versatile toolkit to broaden the adoption of coronary physiology in daily practice.
Philips offers one of the most comprehensive image-guided therapy portfolios in the industry, providing solutions for visual guidance during minimally invasive procedures and image-guided therapy devices designed to enhance procedural efficiency and improve patient outcomes. At the heart of this portfolio is Azurion, Philips’ next-generation platform that brings advanced interventional tools together in a single, intuitive environment supporting a wide range of procedures across interventional cardiology, interventional radiology, neuroradiology and vascular surgery. Introduced in 2017, Philips Azurion is used to treat more than 7.6 million patients each year in over 80 countries.
Financial terms of the agreement are not disclosed.
- The SpectraWAVE HyperVue Imaging System and the Starlight Imaging Catheter is a US Class 2 device cleared by FDA through K221257 and K230691. The SpectraWAVE X1-FFR Software is a US Class 2 device cleared by FDA through K251355.
- Compared with angiography-guided Percutaneous Coronary Interventions.
- AI-derived algorithms are deployed on the device to support vessel segmentation and contouring. Analytical (non-AI) models are used for generating FFR values.
- Stark, B., Johnson, C., & Roth, G. A. (2024). Global prevalence of coronary artery disease: An update from the Global Burden of Disease Study. Journal of the American College of Cardiology, 83(13 Suppl), 2320. https://doi.org/10.1016/S0735-1097(24)04310-9
- Mandurino-Mirizzi, A., Munafò, A. R., Rizzo, F., De Francesco, R., Raone, L., Germinal, F., Montalto, C., Mussardo, M., Moci, M., Vergallo, R., Rocco, V., Fischetti, D., Dionigi, F., Godino, C., Colonna, G., Oreglia, J., Burzotta, F., Crimi, G., & Porto, I. (2025). Comparison of different guidance strategies to percutaneous coronary intervention: A network meta-analysis of randomized clinical trials. International Journal of Cardiology, 422, 132936. https://doi.org/10.1016/j.ijcard.2024.132936
- Stone, G. W., Christiansen, E. H., Ali, Z. A., Andreasen, L. N., Maehara, A., Ahmad, Y., Landmesser, U., & Holm, N. R. (2024). Intravascular imaging-guided coronary drug-eluting stent implantation: An updated network meta-analysis. The Lancet, 403(10429), 824–837. https://doi.org/10.1016/S0140-6736(23)02454-6
- Gaster, A. L., Slothuus Skjoldborg, U., Larsen, J., Korsholm, L., von Birgelen, C., Jensen, S., Thayssen, P., Pedersen, K. E., & Haghfelt, T. H. (2003). Continued improvement of clinical outcome and cost effectiveness following intravascular ultrasound guided PCI: Insights from a prospective, randomized study. Heart, 89(9), 1043–1049. https://doi.org/10.1136/heart.89.9.1043


 October 24, 2025
October 24, 2025