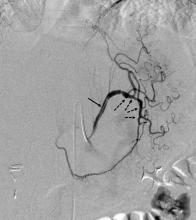
June St. Jude Medical Inc. announced preliminary results from the EnligHTN III study, which found the company’s second-generation EnligHTN renal denervation system provided safe and effective therapy for patients with drug-resistant, uncontrolled hypertension six months post-procedure.
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now