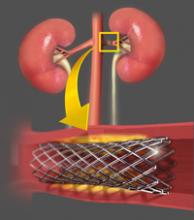
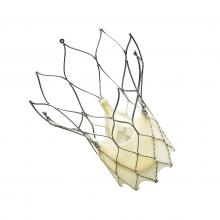
May 21, 2014 — Siemens Healthcare announced that the U.S. Food and Drug Administration (FDA) cleared the Somatom Force computed tomography (CT) system — the next generation in dual source CT.
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now