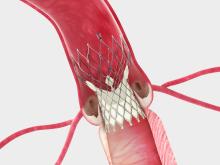
Transcatheter Technologies GmbH, an emerging medical device company that is developing a third-generation transcatheter aortic valve implantation (TAVI) system — Trinity) — announced that its founder and CEO, Wolfgang Goetz, M.D., Ph.D., will be meeting with potential investors, including potential corporate partners, at the EuroPCR annual scientific meeting in Paris, May 20-23.
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now