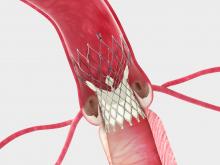
Patients with peripheral artery disease in the upper leg experienced significantly better outcomes at 12 months after treatment with the IN.PACT Admiral drug-coated balloon from Medtronic Inc. than with standard balloon angioplasty, according to a landmark clinical study reported on today for the first time.
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now