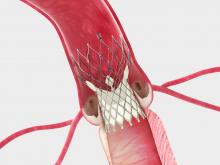
April 23, 2014 — Xeltis announced it finished enrollment in a five-patient feasibility study of implantable products intended to enable for the first time the spontaneous growth of natural, healthy heart valves and vessels.
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now