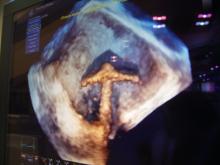
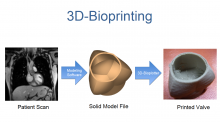
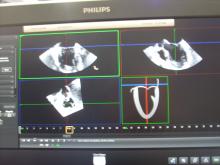
The Cardiovascular Research Foundation’s Transcatheter Cardiovascular Therapeutics (TCT) 2013 offers many new insights about the latest cardiovascular technologies and treatment techniques. These are my choices for the most innovative new or futuristic technologies highlighted on the show floor or in sessions at TCT 2013.
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now