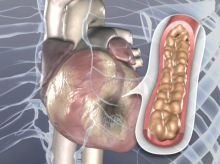
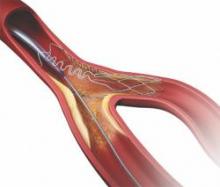
According to a new study, genetic profiling of patients undergoing percutaneous coronary intervention (PCI) may help cardiology teams adjust treatment and improve ischemic outcomes for patients who do not properly metabolize thienopyridine blood thinning therapies such as clopidogrel.
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now