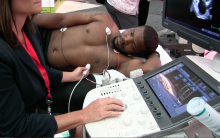
March 7, 2013 — Ultrasound technology could soon experience a significant upgrade that would enable it to produce high-quality, high-resolution images, thanks to the development of a new key material by a team of researchers that includes a professor in the department of biomedical engineering at Texas A&M University.
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now