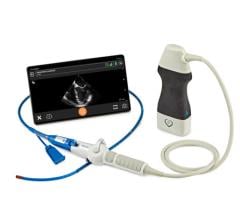
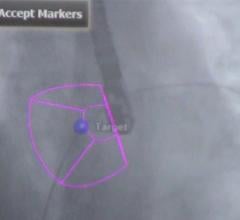
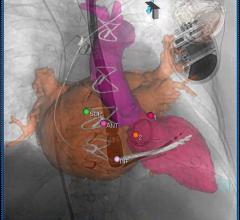
March 7, 2013 — Philips Healthcare announced it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its EchoNavigator live image-guidance tool. The technology helps interventional cardiologists and cardiac surgeons perform minimally invasive structural heart disease repairs by providing an intelligently integrated view of live X-ray and 3-D ultrasound images.
Following the CE marking of EchoNavigator in Europe, Philips will now be able to introduce the system globally, with systems already installed in Europe and the United States.
EchoNavigator was developed in response to an upward trend in the use of both X-ray imaging and 3-D cardiac ultrasound imaging (echocardiography) during structural heart disease procedures — an area of interventional cardiology that is growing at around 40 percent per year. During such procedures, ultrasound imaging provides critical insights into the heart’s soft tissue anatomy, while X-ray imaging has particular strengths in visualizing the catheters and heart implants. EchoNavigator was designed to address the unique challenges associated with working with live X-ray and 3-D ultrasound images simultaneously.
"Together with Philips, we set out to bring two separate medical imaging techniques together in a way that provides clear visual guidance," said John Carroll, M.D., interventional cardiologist, University of Colorado Hospital, Denver. "EchoNavigator is enabling us to use X-ray images combined with real-time 3-D ultrasound images to navigate catheters and deploy implants in the right position in the heart, making such treatments more straightforward."
EchoNavigator will enable clinicians to perform procedures more efficiently by providing intelligently integrated X-ray and 3-D ultrasound images into one intuitive and interactive view, as well as providing easy-to-use system navigation and better communication between the multidisciplinary team carrying out the procedure.
“We have learned that ideally two live imaging technologies are needed to guide catheter-based repairs to the heart, and a multidisciplinary team is needed to perform it,” said Roberto Corti, M.D., interventional cardiologist, University Hospital Zurich, Switzerland. “This adds to the complexity of such procedures. The development of a more sophisticated imaging technology such as EchoNavigator will definitely provide us with a better understanding of the complex structures of the heart and their repair.”
“As the global market leader in interventional cardiology, we have worked with our partners to lead the way with pioneering solutions such as our real-time 3-D ultrasound technology and more recently our HeartNavigator navigation tool," said Gene Saragnese, CEO for Imaging Systems at Philips Healthcare. "EchoNavigator is further evidence of our commitment to transforming healthcare through the introduction of innovations that enable best in class minimally invasive procedures."
“In the emerging field of complex structural heart disease interventions, the information obtained by merging imaging technologies, as now possible with HeartNavigator and EchoNavigator, will be of tremendous value to the interventionalist, and in turn to the patient,” said Carlos Ruiz, M.D., director of the structural and congenital heart disease program, department of interventional cardiology, Lenox Hill Hospital, New York.
For more information: www.healthcare.philips.com


 June 13, 2024
June 13, 2024