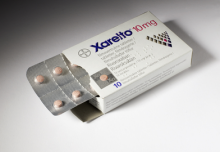
June 22, 2012 — The the U.S. Food and Drug Administration’s (FDA) Cardiovascular and Renal Drugs Advisory Committee voted in May against recommending approval of expanding the indication for rivaroxaban (Xarelto) for the reduction of the risk of secondary cardiovascular events in patients with acute coronary syndrome (ACS).
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now