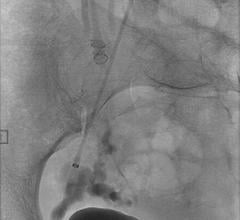
June 21, 2012 — Surefire Medical Inc. announced that it has received 510(k) U.S. Food and Drug Administration (FDA) clearance to market the Surefire high-flow microcatheter, the next generation of the company's infusion technology. The Surefire system is designed to deliver therapy with higher infusion efficiency than conventional microcatheters.
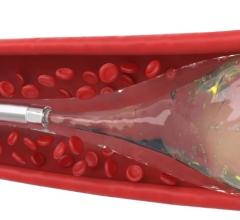
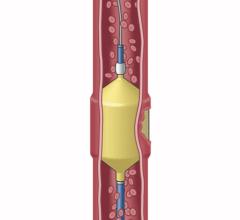
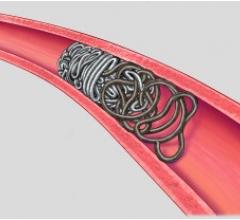
The Surefire microcatheter's expandable tip dynamically expands in reverse flow and collapses in forward flow.
Pre-clinical study results presented at the World Congress of Interventional Oncology (WCIO) in June 2011 found that the Surefire infusion system achieved an average infusion efficiency of 99.1 percent compared to 72.8 percent with a standard infusion catheter.
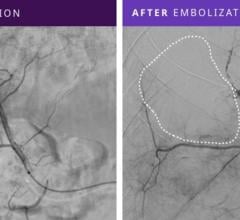
In addition, an abstract presented by John Louie, M.D. of Stanford University during the 2012 Annual Scientific Meeting for Society of Interventional Radiology (SIR) stated, "In chemoembolization patients, completion C-arm CT imaging suggested a pattern of increased microsphere tumor penetration and contrast retention. In short term follow-up, no clinical evidence of non-target embolization was observed."
Since launching in August 2011, the initial Surefire infusion system has been used in 68 high-volume, top-tier hospitals, primarily in interventional procedures to treat primary and secondary liver cancer.
Surefire's infusion catheters precisely deliver embolic agents through a novel expandable tip that collapses in forward flow and expands to the vessel wall in reverse flow. The Surefire infusion system is designed to increase infusion efficiency and minimize reflux.
For more information: www.surefiremedical.com


 June 05, 2025
June 05, 2025