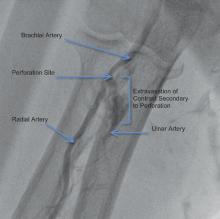
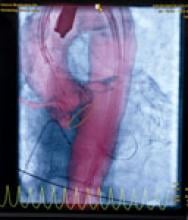
The radial artery approach is exceptionally well-suited for diagnostic angiography and, with a lower incidence of bleeding complications, is a favorable alternative for femoral percutaneous coronary intervention (PCI). The most common complications with the transradial approach are asymptomatic radial artery occlusion (3-5 percent) and radial artery perforation (less than 1 percent).
If you enjoy this content, please share it with a colleague
- Read more about How to Contend With Transradial Artery Perforation
- Log in or register to post comments