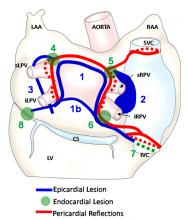
November 23, 2011 –Varian Medical Systems will exhibit its PaxScan digital image detectors and new PaxPower X-ray tubes at the MEDICA 2011 International Trade Fair in Dusseldorf, Germany from November 16-19, 2011.
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now