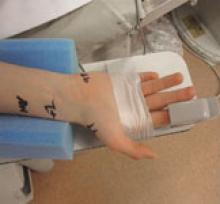
The femoral artery approach for cardiac catheterizations has been used as the gold-standard in the United States, but there is growing interest in the transradial approach. One of the main advantages of radial access is increased safety with fewer bleeding complications.
If you enjoy this content, please share it with a colleague
- Read more about Tips When Prepping for Radial Procedures
- Log in or register to post comments