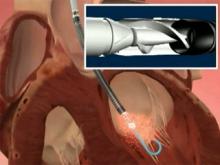
October 5, 2010 – A U.S. clinical study evaluating the CrossBoss and Stingray catheters in treating chronically occluded coronary arteries concluded in August and Bridgepoint Medical has asked the Food and Drug Administration (FDA) to expand the indication for the devices.
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now