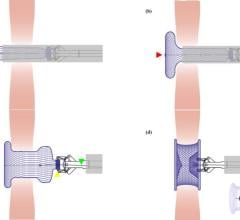
October 4, 2010 – Germany will reimburse patients who have a left atrial appendage closure procedure in 2011. The 2011 G-DRG (German Diagnosis Related Group) catalog, recently published on the InEK website, adds the left atrial appendage closure procedure to an existing G-DRG code.
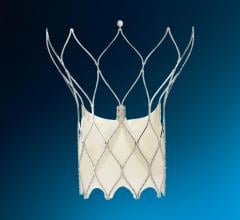
The coverage applies to Atritech’s Watchman, an alternative to long-term warfarin therapy. The Watchman is the only left atrial appendage closure device that has been studied under a multi-center, prospective randomized clinical study.
The most recent data from the PROTECT AF study, presented at the Transcatheter Cardiovascular Therapeutics (TCT) 2010 symposium in Washington, D.C. showed a relative risk reduction of 30 percent for stroke, cardiovascular death and systemic embolism compared to long-term warfarin therapy.
For more information: www.atritech.net


 June 20, 2024
June 20, 2024