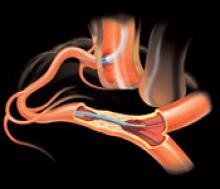
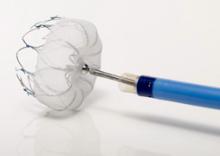
Following cath lab procedures, the final step is to attain hemostasis at the arteriotomy access site. Manual compression has been the gold standard for decades, but vascular closure devices (VCDs) can speed wound closure, and hemostatic pads can help decrease clotting times. VCDs and the new dressings also accelerate patient ambulation.
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now