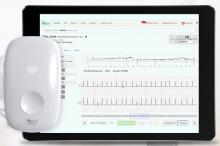
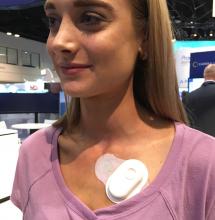
Philips will showcase its latest cardiac care innovations at the European Society of Cardiology (ESC) Congress 2019, Aug. 31–Sept. 4 in Paris, France. At the congress, Philips is showcasing Release 5.0 of its Epiq CVx cardiology platform for the first time in Europe. The platform includes automated applications for 2-D assessment of the heart, as well as robust 3-D right ventricle volume and ejection fraction measurements, making accurate exams faster and easier to conduct. Philips also announced that it is collaborating with digital health company LindaCare to combine the latter’s OnePulse cloud-based solution for the remote monitoring of patients with cardiac implantable electronic devices (CIEDs) with the Philips IntelliSpace Cardiovascular informatics platform.
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now