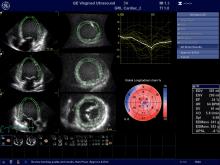
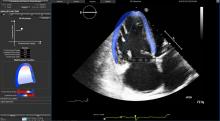
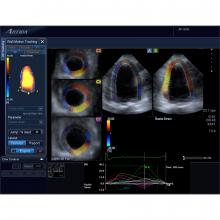
Cardio-oncology is an emerging field that combines the expertise of both cardiology and oncology to assess and treat cancer patients for the second leading cause of death among cancer survivors — cardiovascular disease brought on by their treatments. Specific types of chemotherapy and chest-directed radiation therapy are known to cause cardiac dysfunction, mainly due to cardiotoxicity — the symptoms of which may not present until months or even years after cancer treatment.
If you enjoy this content, please share it with a colleague
- Read more about Assessing Cardiotoxicity Due to Cancer Therapy
- Log in or register to post comments