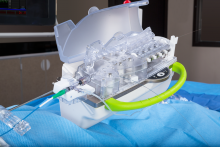
NuCryo Vascular LLC announced the launch of the Next Generation Cryoplasty Inflation device. The device received U.S. Food and Drug Administration (FDA) 510(k) clearance in late 2015 and works in conjunction with the PolarCath Balloon Dilatation System.
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now